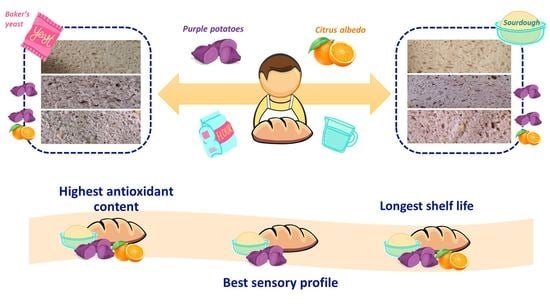
Bread Fortified with Cooked Purple Potato Flour and Citrus Albedo: An Evaluation of Its Compositional and Sensorial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Material
2.3. Bread Preparation
2.4. Chemical Composition of Bread
2.5. Colour Parameters
2.6. Phenolic Compounds and Antioxidant Properties of Breads
2.6.1. Extract Preparation
2.6.2. UHPLC-DAD-HR-ESI-MS/MS Analysis
2.6.3. Total Phenol Evaluation
2.6.4. Total Anthocyanin Evaluation
2.6.5. Determination of Anti-Radical Activity
2.7. Volatile Organic Compound (VOCs) Profile of Bread
2.8. Sensory Characterization of Bread (Crust and Crumb)
2.9. Bread Shelf-Life Assessment
2.10. Statistical Analysis
3. Results
3.1. Physical-Chemicals Parameters
3.1.1. Main Ingredients’ Characterization
3.1.2. Cooked Bread
3.2. Phenolic Compounds and Antioxidant Properties of Breads
3.3. Volatile Bouquet in the Headspace Emissions of Whole and Sliced Breads
3.4. Sensorial Parameters
3.5. Shelf Life: Preliminary Results
- kc = kinetic constant (mm·days−1)
- t = time (days)
- qc = (mm)
- kw = kinetic constant (days−1)
- t = time (days).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Smith, J.P.; Daifas, D.P.; El-Khoury, W.; Koukoutsis, J.; El-Khoury, A. Shelf life and safety concerns of bakery products–A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 19–55. [Google Scholar] [CrossRef]
- Taglieri, I.; Macaluso, M.; Bianchi, A.; Sanmartin, C.; Quartacci, M.F.; Zinnai, A.; Venturi, F. Overcoming bread quality decay concerns: Main issues for bread shelf life as a function of biological leavening agents and different extra ingredients used in formulation. A review. J. Sci. Food Agric. 2021, 101, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread staling: Updating the view. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Katina, K. Sourdough: A Tool for the Improved Flavour, Texture and Shelf-Life of Wheat Bread; VTT Publications: Espoo, Finland, 2005; ISBN 4567890123456. [Google Scholar]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of Sourdough Technology: From Production to Marketing. Food Bioprocess Technol. 2017, 11, 242–270. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Andrich, G. Changes in physicochemical and sensory characteristics of fresh bread rolls maintained in different storage conditions. Agrochimica 2012, 56, 140–155. [Google Scholar]
- Behera, S.S.; Ray, R.C. Sourdough bread. In Bread: Its Fortification for Nutrition and Health; Cristina, M.R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 53–67. [Google Scholar]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Zinnai, A. Effect of the baking process on artisanal sourdough bread-making: A technological and sensory evaluation. Agrochimica 2016, 60, 222–234. [Google Scholar]
- Arendt, E.K.; Ryan, L.A.; Bello, F.D. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Gocmen, D.; Gürbüz, O.; Kumral, A.Y.; Dagdelen, A.F.; Şahin, I. The effects of wheat sourdough on glutenin patterns, dough rheology and bread properties. Eur. Food Res. Technol. 2007, 225, 821–830. [Google Scholar] [CrossRef]
- Katina, K.; Heiniö, R.-L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT-Food Sci. Technol. 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Torrieri, E.; Pepe, O.; Ventorino, V.; Masi, P.; Cavella, S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT-Food Sci. Technol. 2014, 56, 508–516. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. Stone milling versus roller milling: A systematic review of the effects on wheat flour quality, dough rheology, and bread characteristics. Trends Food Sci. Technol. 2020, 97, 147–155. [Google Scholar] [CrossRef]
- Arufe, S.; Chiron, H.; Doré, J.; Savary-Auzeloux, I.; Saulnier, L.; Della Valle, G. Processing & rheological properties of wheat flour dough and bread containing high levels of soluble dietary fibres blends. Food Res. Int. 2017, 97, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.; Simsek, S. Potato flour as a functional ingredient in bread: Evaluation of bread quality and starch characteristics. Int. J. Food Sci. Technol. 2020, 55, 3639–3649. [Google Scholar] [CrossRef]
- Burgos, G.; Felde, T.Z.; Andre, C.; Kubow, S. The potato and its contribution to the human diet and health. In The Potato Crop; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 37–74. [Google Scholar]
- Gumul, D.; Ziobro, R.; Ivanišová, E.; Korus, A.; Árvay, J.; Tóth, T. Gluten-free bread with an addition of freeze-dried red and purple potatoes as a source of phenolic compounds in gluten-free diet. Int. J. Food Sci. Nutr. 2016, 68, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Santiago, D.M.; Matsushita, K.; Noda, T.; Tsuboi, K.; Yamada, D.; Murayama, D.; Koaze, H.; Yamauchi, H. Effect of purple sweet potato powder substitution and enzymatic treatments on bread making quality. Food Sci. Technol. Res. 2015, 21, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-G.; Yan, Q.-Q.; Xue, R.-Y.; Zhang, J.; Zhang, Y.-Q. Isolation and identification of colourless caffeoyl compounds in purple sweet potato by HPLC-DAD–ESI/MS and their antioxidant activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef]
- Sgherri, C.; Micaelli, F.; Andreoni, N.; Baldanzi, M.; Ranieri, A. Retention of phenolic compounds and antioxidant properties in potato bread obtained from a dough enriched with a powder from the purple cv. Vitelotte. Agrochimica 2016, 60, 312–328. [Google Scholar] [CrossRef]
- Tierno, R.; López, A.; Riga, P.; Arazuri, S.; Jarén, C.; Benedicto, L.; Galarreta, J.I.R.D. Phytochemicals determination and classification in purple and red fleshed potato tubers by analytical methods and near infrared spectroscopy. J. Sci. Food Agric. 2015, 96, 1888–1899. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Na Kim, S.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [Green Version]
- Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnai, A.; Mensuali, A. Potato peels as a source of novel green extracts suitable as antioxidant additives for fresh-cut fruits. Appl. Sci. 2019, 9, 2431. [Google Scholar] [CrossRef] [Green Version]
- Wirkijowska, A.; Zarzycki, P.; Sobota, A.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. The possibility of using by-products from the flaxseed industry for functional bread production. LWT 2020, 118, 108860. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobedo-Avellaneda, Z.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; Torres, J.A.; Welti-Chanes, J. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J. Funct. Foods 2014, 6, 470–481. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Ferrero, C. Hydrocolloids in wheat breadmaking: A concise review. Food Hydrocoll. 2017, 68, 15–22. [Google Scholar] [CrossRef]
- Sivam, A.; Sun-Waterhouse, D.; Perera, C.; Waterhouse, G. Application of FT-IR and Raman spectroscopy for the study of biopolymers in breads fortified with fibre and polyphenols. Food Res. Int. 2013, 50, 574–585. [Google Scholar] [CrossRef]
- Correa, M.J.; Pérez, G.T.; Ferrero, C. Pectins as breadmaking additives: Effect on dough rheology and bread quality. Food Bioprocess. Technol. 2011, 5, 2889–2898. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Serra, A.; Conte, G.; Flamini, G.; Angelini, L.G. Effect of the leavening agent on the compositional and sensorial characteristics of bread fortified with flaxseed cake. Appl. Sci. 2020, 10, 5235. [Google Scholar] [CrossRef]
- AACC. AACC Method 44-15.02 Moisture—Air-Oven Methods. In AACC Approved Methods of Analysis; General and Grains: Chiasso, Switzerland, 2000. [Google Scholar]
- AACC. AACC Method 02-52.01. Hydrogen-Ion Activity (pH)—Electrometric Method. In AACC Approved Methods of Analysis; General and Grains: Chiasso, Switzerland, 2000. [Google Scholar]
- Gelinas, P.; Audet, J.; Lachance, O.; Vachon, M. Fermented dairy ingredients for bread: Effects on dough rheology and bread characteristics’. Cereal Chem. 1995, 72, 151–154. [Google Scholar]
- Beutler, H.O. Ethanol. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publisher (UK) Ltd.: Cambridge, UK, 1988; pp. 598–606. [Google Scholar]
- Beutler, H.O. Determination with Acetyl-CoA Synthetase. In Methods of Enzymatic Analysis; Bermeyer, H.-U., Ed.; VCH Publishers (UK) Ltd.: Cambridge, UK, 1988; pp. 639–645. [Google Scholar]
- Noll, F. L-(+)-lactate. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers (UK) Ltd.: Cambridge, UK, 1988; pp. 582–588. [Google Scholar]
- Gawehn, K. D-(-)-lactate. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Publishers (UK) Ltd.: Cambridge, UK, 1988; pp. 588–592. [Google Scholar]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef] [Green Version]
- Macaluso, M.; Bianchi, A.; Sanmartin, C.; Taglieri, I.; Venturi, F.; Testai, L.; Flori, L.; Calderone, V.; De Leo, M.; Braca, A.; et al. By-products from winemaking and olive mill value chains for the enrichment of refined olive oil: Technological challenges and nutraceutical features. Foods 2020, 9, 1390. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.; Piragine, E.; Pirone, A.; Braca, A.; Pistelli, L.; Calderone, V.; Miragliotta, V.; Testai, L. Protective effects of bergamot (Citrus bergamia Risso & Poiteau) Juice in Rats Fed with High-Fat Diet. Planta Medica. 2019, 86, 180–189. [Google Scholar] [CrossRef]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadhani, M.B.; Patel, V.H.; Subhash, R. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef] [Green Version]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable Sensors for Assessing the Role of Olfactory Training on the Autonomic Response to Olfactory Stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef]
- Al Omari, D.; Abdul-Hussain, S.; Ajo, R. Germinated lupin (Lupinus albus) flour improves Arabic flat bread properties. Qual. Assur. Saf. Crop. Foods 2016, 8, 57–63. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hort, J.; Hollowood, T. Descriptive analysis in Sensory Evaluation, 1st ed.; Kemp, S.E., Hort, J., Hollowood, T., Eds.; John Wiley & Sons Ltd. All: Hoboken, NJ, USA, 2018; ISBN 9781118991671. [Google Scholar]
- Brun, R.; Rademakers, F. ROOT—An object oriented data analysis framework. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1997, 389, 81–86. [Google Scholar] [CrossRef]
- Sánchez-Pardo, M.E.E.; Blancas-Nápoles, J.A.A.; Vázquez-Landaverde, P.A.A.; Nari, A.; Taglieri, I.; Ortiz-Moreno, A.; Mayorga-Reyes, L.; Sanmartin, C.; Bermúdez-Humarán, L.G.G.; Torres-Maravilla, E. The use of Mexican xaxtle as leavening agent in Italian straight dough bread making to produce pulque bread. Agrochimica 2016, 60, 329–342. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative Activity of Anthocyanins from Purple Sweet Potato, Ipomoera batatas Cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin Composition of Black Carrot (Daucus carota ssp. sativus var. atrorubens Alef.) Cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Saa, D.L.T.; Gianotti, A. Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Gerez, C.L.; Mohtar, L.G.M.; Zanini, V.I.P.; Nazareno, M.A.; Taranto, M.P.; Iturriaga, L.B. Lactic Acid Fermentation Improved Textural Behaviour, Phenolic Compounds and Antioxidant Activity of Chia (Salvia hispanica L.) Dough. Food Technol. Biotechnol. 2017, 55, 381–389. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J. Chromatogr. A 2004, 1054, 143–155. [Google Scholar] [CrossRef]
- Eichhorn, S.; Winterhalter, P. Anthocyanins from pigmented potato (Solanum tuberosum L.) varieties. Food Res. Int. 2005, 38, 943–948. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Andrenelli, L.; Vecchio, V.; Mulinacci, N. Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chem. 2011, 125, 750–759. [Google Scholar] [CrossRef]
- Lewis, C.E.; John, R.L.W.; Jane, E.L.; Kevin, H. Sutton. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. II: Wild, tuberous Solanum species. J. Sci. Food Agric. 1998, 77, 58–63. [Google Scholar] [CrossRef]
- Mencherini, T.; Campone, L.; Piccinelli, A.L.; Mesa, M.G.; Sánchez, D.M.; Aquino, R.P.; Rastrelli, L. HPLC-PDA-MS and NMR Characterization of a Hydroalcoholic Extract of Citrus aurantium L. var. amara Peel with Antiedematogenic Activity. J. Agric. Food Chem. 2013, 61, 1686–1693. [Google Scholar] [CrossRef]
- Yu, L.; Chen, M.; Liu, J.; Huang, X.; He, W.; Qing, Z.; Zeng, J. Systematic detection and identification of bioactive ingredients from Citrus aurantium L. var. amara using HPLC-Q-TOF-MS combined with a screening Method. Molecules 2020, 25, 357. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Bao Phenolic Compounds and Antioxidant Activities of Potato Cultivars with White, Yellow, Red and Purple Flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Akter, M.S.; Lee, J.-C.; Eun, J.-B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Orsák, M.; Hamouz, K.; Lachman, J.; Kasal, P. Chlorogenic acid content in potato tubers with colored flesh as affected by a genotype, location and long-term storage. Plant Soil Environ. 2019, 65, 355–360. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.-J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitrocolonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein–flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef]
- Yuan, W.; Fan, W.; Mu, Y.; Meng, D.; Yan, Z.; Li, Y.; Lv, Z. Baking intervention for the interaction behaviours between bamboo (Phyllostachys heterocycla) leaf flavonoids and gliadin. Ind. Crop. Prod. 2021, 164, 113385. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; Van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Pęksa, A.; Kita, A.; Kułakowska, K.; Aniołowska, M.; Hamouz, K.; Nemś, A. The quality of protein of coloured fleshed potatoes. Food Chem. 2013, 141, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Pęksa, A.; Miedzianka, J.; Nemś, A. Amino acid composition of flesh-coloured potatoes as affected by storage conditions. Food Chem. 2018, 266, 335–342. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Sun, F.; Han, H.; Zhang, X.; Fan, Y.; Tai, G.; Zhou, Y. In vivo antimalarial activities of glycoalkaloids isolated from Solanaceae plants. Pharm. Biol. 2010, 48, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Friedman, M.; McDonald, G.M. Postharvest Changes in Glycoalkaloid Content of Potatoes. In Impact of Processing on Food Safety; Advances in Experimental Medicine and, Biology; Jackson, L.S., Knize, M.G., Morgan, J.N., Eds.; Springer: Boston, MA, USA, 1999; Volume 459, pp. 121–143. [Google Scholar]
- Tajner-Czopek, A.; Rytel, E.; Kita, A.; Pęksa, A.; Hamouz, K. The influence of thermal process of coloured potatoes on the content of glycoalkaloids in the potato products. Food Chem. 2012, 133, 1117–1122. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Human Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Ainee, A.; Nadeem, M.; Munir, M.; Qureshi, T.M.; Jabbar, S. Effect of Grape Fruit Albedo Powder on the Physicochemical and Sensory Attributes of Fruit Cake. Pak. J. Agric. Res. 2017, 30, 185–193. [Google Scholar] [CrossRef]
- Hassan, M.A.M.; Ali, H.M. Physico-chemical properties and sensory evaluation of toast bread fortified with different levels of white grapefruit (Citrus paradise L.) albedo layer flour. World J. Dairy Food Sci. 2014, 9, 228–234. [Google Scholar] [CrossRef]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Fabroni, S.; Fallico, B.; Rapisarda, P.; Arena, E. Wholegrain durum wheat bread fortified with citrus fibers: Evaluation of quality parameters during long storage. Front. Nutr. 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Ajila, C.; Leelavathi, K.; Rao, U.P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Rosell, C.; Rojas, J.; De Barber, C.B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Han, L.; Zhang, J.; Cao, X. Effects of orange peel powder on rheological properties of wheat dough and bread aging. Food Sci. Nutr. 2021, 9, 1061–1069. [Google Scholar] [CrossRef] [PubMed]










| Weak Wheat Flour | Water | S Biga | Y Biga | Cooked Purple Potato Flour | Citrus Albedo | |
|---|---|---|---|---|---|---|
| SB0 | 52% | 32% | 16% | - | - | - |
| SB+P | 44% | 32% | 16% | - | 8% | - |
| SB+PA | 43.25% | 32% | 16% | - | 8% | 0.75% |
| YB0 | 52% | 32% | - | 16% | - | - |
| YB+P | 4% | 32% | - | 16% | 8% | - |
| YB+PA | 43.25% | 32% | - | 16% | 8% | 0.75% |
| p-Value 1 | Weak Wheat Flour | Lyophilized Purple Potatoes (P) | Lyophilized Albedo (A) | |
|---|---|---|---|---|
| Dry matter (% dm) | *** | 89.38 b | 95.31 a | 86.34 c |
| Total phenols (mg GAE/g dm) | *** | 0.25 c | 5.38 b | 42.71 a |
| Antiradical activity (µmol TE/g dm) | *** | 0.993 c | 51.448 b | 85.06 a |
| TEAC (µmol TE/g dm) | *** | 0.504 c | 27.248 b | 43.637 a |
| Total anthocyanins (mg cyanidin-3-O-glycoside/kg dm) | *** | b.d.l | 2263 a | 33 b |
| p-Value 1 | Sourdough Biga | Baker’s Yeast Biga | |
|---|---|---|---|
| Dry matter (% dm) | *** | 57.7 b | 61.8 a |
| pH | *** | 3.84 b | 5.84 a |
| Total titratable acidity (meq lactic acid/g dm) | *** | 0.102 a | 0.008 b |
| Acetic acid (mmol/g dm) | * | 0.043 a | 0.037 b |
| d-Lactic acid (mmol/g dm) | *** | 0.026 a | b.d.l 2 |
| l-Lactic acid (mmol/g dm) | *** | 0.102 a | b.d.l. |
| Ethanol (mmol/g dm) | ** | 0.113 b | 0.308 a |
| p-Value 1 | SB0 | SB+P | SB+PA | YB0 | YB+P | YB+PA | |
|---|---|---|---|---|---|---|---|
| Water activity | n.s./§§§/¶¶ | 0.958 a | 0.955b | 0.952 c | 0.958 a | 0.954 b | 0.953 c |
| Dry matter (%) | n.s./***/n.s. | 57.3 | 54.0 | 55.5 | 57.3 | 53.4 | 55.3 |
| pH | ***/§§§/¶¶¶ | 4.10 e | 4.61 c | 4.32 d | 5.86 b | 6.06 a | 5.78 b |
| TTA (meq lactic acid/g dm) | ***/§§§/¶¶¶ | 0.044 b | 0.043 b | 0.062 a | 0.006 d | 0.008 d | 0.012 c |
| Acetic acid (mmol/g dm) | n.s./n.s./n.s. | 0.061 | 0.045 | 0.043 | 0.044 | 0.041 | 0.050 |
| d-Lactic acid (mmol/g dm) | ***/§§§/¶¶¶ | 0.011 b | 0.019 a | 0.020 a | b.d.l. 2 | b.d.l. | b.d.l. |
| l-Lactic acid (mmol/g dm) | ***/§§§/¶¶¶ | 0.067 b | 0.079 a | 0.083 a | b.d.l. | b.d.l. | b.d.l. |
| Ethanol (mmol/g dm) | ***/§/¶ | 0.032 c | 0.059 b | 0.051 b | 0.040 c | 0.084 a | 0.087 a |
| p-Value 1 | SB0 | SB+P | SB+PA | YB0 | YB+P | YB+PA | |
|---|---|---|---|---|---|---|---|
| L* | n.s./§§§/¶¶¶ | 66.62 a | 57.75 c | 56.46 c | 68.97 a | 60.64 b | 52.18 d |
| a* | ***/§§§/¶¶¶ | 0.69 c | 5.98 a | 5.66 a | 0.29 c | 3.53 b | 3.79 b |
| b* | n.s./§§§/¶¶¶ | 15.92 a | 7.53 e | 11.28 c | 14.69 b | 8.60 d | 11.82 c |
| H* | ***/§§§/¶¶¶ | 1.53 a | 0.90 e | 1.11 d | 1.55 a | 1.18 c | 1.26 b |
| C* | ***/§§§/¶¶ | 15.94 a | 9.61 d | 12.62 c | 14.69 b | 9.30 d | 12.41 c |
| SB0 | SB+P | SB+PA | YB0 | YB+P | YB+PA | |
|---|---|---|---|---|---|---|
| SB0 | 13.30 | 12.22 | 2.67 | 9.87 | 15.32 | |
| SB+P | 3.98 | 14.47 | 3.93 | 7.37 | ||
| SB+PA | 14.03 | 5.40 | 4.71 | |||
| YB0 | 10.82 | 17.39 | ||||
| YB+P | 9.05 | |||||
| YB+PA |
| p-Value 1 | SB0 | SB+P | SB+PA | YB0 | YB+P | YB+PA | |
|---|---|---|---|---|---|---|---|
| Total phenols (mg GAE/g dm) | ***/§§§/¶ | 0.867 d | 1.571 b | 2.304 a | 0.589 e | 1.273 c | 1.684 b |
| Total anthocyanins (mg cyanidin-3-O-glycoside/kg dm) | ***/§§§/n.s. | b.d.l. | 264 | 237 | b.d.l. | 230 | 217 |
| DPPH (μmol TE/g dm) | ***/§§/¶ | 1.521 d | 5.766 b | 6.583 a | 1.493 d | 4.835 c | 5.644 b |
| TEAC (μmoL TE/g dm) | ***/§§§/¶ | 0.843 d | 2.885 b | 3.195 a | 0.848 d | 2.498 c | 2.885 b |
| Sensory Parameters | p-Value 1 | SB0 | SB+P | SB+PA | YB0 | YB+P | YB+PA | |
|---|---|---|---|---|---|---|---|---|
| Crumb | ||||||||
| Crumb colour intensity (White scale) | *** | 5.25 a | 0.00 b | 0.00 b | 4.48 a | 0.00 b | 0.00 b | |
| Crumb colour intensity (Purple scale) | *** | 0.00 b | 4.08 a | 4.30 a | 0.00 b | 2.28 ab | 2.88 ab | |
| Presence of rips | *** | 1.63 a | 0.58 b | 0.98 ab | 1.97 a | 0.43 b | 0.73 b | |
| Alveoli dimension | * | 3.10 a | 3.13 a | 2.33 a | 1.95 b | 1.83 b | 1.13 b | |
| Homogeneity of alveolation | * | 4.45 b | 4.50 b | 4.88 b | 5.18 ab | 6.02 a | 6.63 a | |
| Smell intensity | * | 6.63 a | 4.70 ab | 4.60 ab | 4.58 ab | 3.20 b | 4.15 ab | |
| Wheat smell | * | 3.38 a | 2.73 b | 3.05 ab | 4.38 a | 1.88 b | 2.38 b | |
| Yeast smell | n.s. | 3.33 | 2.08 | 2.28 | 3.50 | 3.98 | 3.55 | |
| Acetic smell | *** | 4.48 a | 1.90 ab | 1.98 ab | 0.38 b | 0.30 b | 1.05 b | |
| Frankness | * | 6.2 ab | 6.38 a | 5.20 b | 7.73 a | 6.75 a | 6.10 ab | |
| Salted taste | *** | 2.83 ab | 2.13 ab | 3.95 a | 1.10 b | 1.25 b | 1.05 b | |
| Acid taste | *** | 3.65 ab | 4.70 ab | 5.03 a | 0.20 c | 0.15 c | 1.68 bc | |
| Bitter taste | *** | 1.93 b | 0.68 b | 6.18 a | 1.20 b | 1.85 b | 5.53 a | |
| Aftertaste | * | 1.43 ab | 1.38 ab | 3.98 a | 0.33 b | 2.25 ab | 3.15 ab | |
| Springiness | n.s. | 5.30 | 5.73 | 4.00 | 5.98 | 6.23 | 6.50 | |
| Humidity of surface | ** | 3.78 ab | 3.88 ab | 4.23 a | 2.40 ab | 1.68 b | 3.30 ab | |
| Crumb residual | n.s. | 1.98 | 2.48 | 1.65 | 1.73 | 1.18 | 0.88 | |
| Resistance to chewing | n.s | 3.80 | 3.30 | 2.73 | 3.83 | 3.45 | 1.83 | |
| Juiciness | n.s. | 2.90 | 4.78 | 3.40 | 2.05 | 1.20 | 2.85 | |
| Adhesiveness | n.s. | 4.10 | 2.15 | 3.63 | 3.25 | 2.63 | 2.25 | |
| Crust | ||||||||
| Crispiness | * | 5.25 ab | 4.20 ab | 7.35 a | 3.50 b | 4.35 ab | 7.03 a | |
| Hardness | n.s. | 3.00 | 2.30 | 4.05 | 3.53 | 3.33 | 3.05 | |
| Smell intensity | n.s. | 5.85 | 5.80 | 5.97 | 5.33 | 5.33 | 4.63 | |
| Salted taste | * | 3.40 a | 3.48 a | 3.50 a | 2.03 b | 1.75 b | 2.38 b | |
| Toasted taste | ** | 3.78 b | 3.55b | 6.95 a | 3.90 b | 4.63 ab | 5.70 ab | |
| Bitter taste | *** | 2.13 b | 2.25b | 5.58 a | 1.98 b | 3.00 b | 4.80 a | |
| Aftertaste | n.s. | 0.68 | 1.08 | 3.13 | 0.88 | 1.88 | 3.25 | |
| SB0 | SB+P | SB+PA | YB0 | YB+P | YB+PA | |
|---|---|---|---|---|---|---|
| t fin (days) | 3 | 4 | 6 | 2 | 3 | 3 |
| aw | 0.949 ± 0.001 | 0.941 ± 0.001 | 0.940 ± 0.005 | 0.949 ± 0.001 | 0.950 ± 0.001 | 0.950 ± 0.005 |
| L* | 65.29 ± 1.61 | 54.60 ± 0.01 | 53.62 ± 1.01 | 68.75 ± 1.82 | 54.18 ± 0.94 | 55.54 ± 3.49 |
| a* | 0.68 ± 0.09 | 5.32 ± 0.01 | 5.03 ± 0.07 | 0.39 ± 0.06 | 3.28 ± 0.44 | 3.06 ± 0.08 |
| b* | 15.64 ± 0.46 | 9.38 ± 0.01 | 12.11 ± 0.16 | 14.71 ± 0.32 | 8.61 ± 0.24 | 11.85 ± 0.06 |
| C* | 15.66 ± 0.46 | 10.78 ± 0.10 | 13.11 ± 0.15 | 14.71 ± 0.33 | 11.11 ± 0.54 | 12.24 ± 0.15 |
| H* | 1.52 ± 0.20 | 1.16 ± 0.07 | 1.18 ± 0.02 | 1.54 ± 0.23 | 1.16 ± 0.07 | 1.32 ± 0.04 |
| Compressibility (mm) | Weight Decrease (%) | ||||
|---|---|---|---|---|---|
| Sample | q (mm) | ||||
| SB0 | −0.43 ± 0.09 | 1.96 ± 0.09 | 0.46 | −1.21 ± 0.01 | 5.5 |
| SB+P | −0.49 ± 0.03 | 2.47 ± 0.12 | 6.71 | −1.19 ± 0.01 | 3.66 |
| SB+PA | −0.44 ±0.04 | 2.20 ± 0.16 | 4.60 | −1.21 ± 0.01 | 0.84 |
| YB0 | −0.62 ± 0.12 | 2.31 ± 0.13 | 1.03 | −1.40 ± 0.04 | 0.01 |
| YB+P | −0.73 ± 0.14 | 3.68 ± 0.27 | 0.70 | −1.30 ± 0.04 | 1.26 |
| YB+PA | −0.63 ± 0.08 | 2.65 ± 0.16 | 0.12 | −1.22 ± 0.01 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Bianchi, A.; Sgherri, C.; Quartacci, M.F.; De Leo, M.; Pistelli, L.; Palla, F.; et al. Bread Fortified with Cooked Purple Potato Flour and Citrus Albedo: An Evaluation of Its Compositional and Sensorial Properties. Foods 2021, 10, 942. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10050942
Taglieri I, Sanmartin C, Venturi F, Macaluso M, Bianchi A, Sgherri C, Quartacci MF, De Leo M, Pistelli L, Palla F, et al. Bread Fortified with Cooked Purple Potato Flour and Citrus Albedo: An Evaluation of Its Compositional and Sensorial Properties. Foods. 2021; 10(5):942. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10050942
Chicago/Turabian StyleTaglieri, Isabella, Chiara Sanmartin, Francesca Venturi, Monica Macaluso, Alessandro Bianchi, Cristina Sgherri, Mike Frank Quartacci, Marinella De Leo, Luisa Pistelli, Fabrizio Palla, and et al. 2021. "Bread Fortified with Cooked Purple Potato Flour and Citrus Albedo: An Evaluation of Its Compositional and Sensorial Properties" Foods 10, no. 5: 942. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10050942