Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Reagents and Solutions
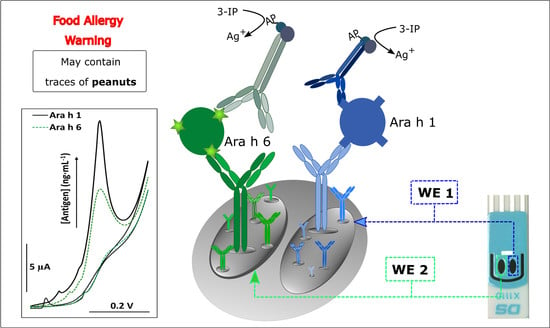
2.3. Immunoassay Strategy
- (i)
- Modification of the working electrodes (WE 1 and WE 2) of the dual-SPCE with the CAbs (5 μL, 25 μg·mL−1), anti-Ara h 1 and anti-Ara h 6, respectively, overnight at 4 °C. Physical adsorption was selected as the immobilization method of the CAbs on the electrode surface due to its simplicity and cost-effectiveness;
- (ii)
- After washing with buffer 1 to remove unbound CAb, the free surface sites were blocked with casein (50 μL, 2% (w/v), 30 min);
- (iii)
- After washing with buffer 1, a mixed standard solution (containing Ara h 1 and Ara h 6) or a diluted sample extract (1000-times dilution) (30 μL, 60 min) was placed on the sensing surface;
- (iv)
- After washing with buffer 1, a mixture of the DAbs (250-times dilutions) and BSA (1% w/v) (30 μL, 30 min) was placed on the dual-SPCE;
- (v)
- After washing with buffer 2 to remove unbound DAb, a 30 μL aliquot of the enzymatic substrate (STR–ALP; 1:150,000) containing BSA (0.1% w/v) was added for 30 min, which was followed by a final washing step with buffer 3;
- (vi)
- Once the affinity events were completed, the enzymatic reaction took place (20 min) by using a mixture (80 μL) of a solution containing 3-IP (1.0 mM) and silver nitrate (0.4 mM) that covered the electrochemical cell.
2.4. Sample Preparation
3. Results and Discussion
3.1. Optimization of the Immunosensor
3.2. Analytical Characteristics
3.3. Sample Analysis
3.4. Comparison with Other Electrochemical Immunoassays for the Analysis of Ara h 1 and Ara h 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, F.; Shi, A.; Ashley, J.; Kronfel, C.; Wang, Q.; Maleki, S.J.; Adhikari, B.; Zhang, J. Peanut Allergy: Characteristics and Approaches for Mitigation. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1361–1387. [Google Scholar] [CrossRef] [Green Version]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiteneder, H.; Peng, Y.-Q.; Agache, I.; Diamant, Z.; Eiwegger, T.; Fokkens, W.J.; Traidl-Hoffmann, C.; Nadeau, K.; O’Hehir, R.E.; O’Mahony, L.; et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 2020, 75, 3039–3068. [Google Scholar] [CrossRef]
- EC. Regulation (EU) No 1169/2011 on the Provision of Food Information to Consumers. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169 (accessed on 18 June 2021).
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freisling, H.; Noh, H.; Slimani, N.; Chajès, V.; May, A.M.; Peeters, P.H.; Weiderpass, E.; Cross, A.J.; Skeie, G.; Jenab, M.; et al. Nut intake and 5-year changes in body weight and obesity risk in adults: Results from the EPIC-PANACEA study. Eur. J. Nutr. 2018, 57, 2399–2408. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Guasch-Ferre, M.; Yanping, L.; Sun, Q.; Willett, W.C.; Tobias, D.K.; Rexrode, K.M.; Drouin-Chartier, J.-P.; Bhupathiraju, S.N. Changes in Nut Consumption and Subsequent Cardiovascular Disease Risk Among US Men and Women: 3 Large Prospective Cohort Studies. J. Am. Heart Assoc. 2020, 9, e013877. [Google Scholar] [CrossRef]
- Mueller, G.A.; Maleki, S.J.; Pedersen, L.C. The Molecular Basis of Peanut Allergy. Curr. Allergy Asthma Rep. 2014, 14, 429. [Google Scholar] [CrossRef] [Green Version]
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852.e7. [Google Scholar] [CrossRef] [PubMed]
- Magnusdottir, H.; Vidarsdóttir, A.G.; Ludviksson, B.R.; Clausen, M.; Lund, S.H.; Jensen, A.B.; Sigurdardottir, S.T. Ara h 1 and Ara h 6 Sensitization Causes Clinical Peanut Allergy in Ara h 2-Negative Individuals. Int. Arch. Allergy Immunol. 2019, 178, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Dreskin, S.C. Redefining the major peanut allergens. Immunol. Res. 2013, 55, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, J.P.M.; Schreurs, M.W.J.; el Bouch, R.; Arends, N.J.T.; de Jong, N.W. Mono-sensitisation to peanut component Ara h 6: A case series of five children and literature review. Eur. J. Pediatr. 2016, 175, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Popping, B.; Diaz-Amigo, C. European Regulations for Labeling Requirements for Food Allergens and Substances Causing Intolerances: History and Future. J. AOAC Int. 2018, 101, 2–7. [Google Scholar] [CrossRef] [PubMed]
- EC. Directive 2003/89/EC of the European Parliament and of the Council of 10 November 2003. Amending Directive 2000/13/EC as Regards Indication of the Ingredients Present in Foodstuffs. OJEU 308(15). 2003. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:308:0015:0018:EN:PDF (accessed on 18 June 2021).
- FDA (Food and Drug Administration). Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA); FDA: Prince George’s County, MD, USA, 2004. [Google Scholar]
- De la Cruz, S.; López-Calleja, I.; Martín, R.; González, I.; Alcocer, M.; García, T. Recent Advances in the Detection of Allergens in Foods. In Food Allergens: Methods and Protocols; Lin, J., Alcocer, M., Eds.; Springer: New York, NY, USA, 2017; pp. 263–295. ISBN 978-1-4939-6925-8. [Google Scholar]
- Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Cutting-Edge Advances in Electrochemical Affinity Biosensing at Different Molecular Level of Emerging Food Allergens and Adulterants. Biosensors 2020, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.S.; Bremer, M.G.E.G.; Nielen, M.W.F. Consumer-friendly food allergen detection: Moving towards smartphone-based immunoassays. Anal. Bioanal. Chem. 2018, 410, 5353–5371. [Google Scholar] [CrossRef] [Green Version]
- Hosu, O.; Selvolini, G.; Marrazza, G. Recent advances of immunosensors for detecting food allergens. Curr. Opin. Electrochem. 2018, 10, 149–156. [Google Scholar] [CrossRef]
- Marques, R.C.B.; Costa-Rama, E.; Viswanathan, S.; Nouws, H.P.A.; Costa-García, A.; Delerue-Matos, C.; González-García, M.B. Voltammetric immunosensor for the simultaneous analysis of the breast cancer biomarkers CA 15-3 and HER2-ECD. Sens. Actuators B Chem. 2018, 255, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Fanjul-Bolado, P.; Hernández-Santos, D.; González-García, M.B.; Costa-García, A. Alkaline Phosphatase-Catalyzed Silver Deposition for Electrochemical Detection. Anal. Chem. 2007, 79, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Silva, T.H.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Improving the extraction of Ara h 6 (a peanut allergen) from a chocolate-based matrix for immunosensing detection: Influence of time, temperature and additives. Food Chem. 2017, 218, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Neves, M.M.P.S.; Nouws, H.P.A.; Santos-Silva, A.; Delerue-Matos, C. Neutrophil Gelatinase-Associated Lipocalin Detection using a Sensitive Electrochemical Immunosensing Approach. Sens. Actuat. B Chem. 2020, 304, 127285. [Google Scholar] [CrossRef]
- Bublin, M.; Breiteneder, H. Cross-reactivity of peanut allergens. Curr. Allergy Asthma Rep. 2014, 14, 426. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Valdepeñas Montiel, V.; Torrente-Rodríguez, R.M.; Campuzano, S.; Pellicanò, A.; Reviejo, Á.J.; Cosio, M.S.; Pingarrón, J.M. Simultaneous Determination of the Main Peanut Allergens in Foods Using Disposable Amperometric Magnetic Beads-Based Immunosensing Platforms. Chemosensors. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Kim, S.W.; Park, C.; Lee, J. Single walled carbon nanotube based biosensor for detection of peanut allergy-inducing protein ara h1. Korean J. Chem. Eng. 2018, 35, 172–178. [Google Scholar] [CrossRef]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance Biosensor for Peanut Protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Lee, J. Detection of Peanut Allergen Ara h 6 in Commercially Processed Foods using a Single-Walled Carbon Nanotube–Based Biosensor. J. AOAC Int. 2018, 101, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Correr, W.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of the peanut allergen Ara h 6 in foodstuffs using a voltammetric biosensing approach. Anal. Bioanal. Chem. 2015, 407, 7157–7163. [Google Scholar] [CrossRef] [PubMed]




| Food/Ingredient | Ara h 1 (mg·g−1) | Ara h 6 (µg·g−1) | ||
|---|---|---|---|---|
| Dual Sensor | ELISA | Dual Sensor | ELISA | |
| Heathy snack | 2.83 ± 0.25 | 2.82 ± 0.09 | 12.0 ± 0.6 | 12.6 ± 0.07 |
| Peanut cookie | 1.37 ± 0.15 | 1.58 ± 0.07 | 12.9 ± 0.7 | 13.1 ± 0.08 |
| Peanut butter | 3.95 ± 0.18 | 3.96 ± 0.08 | 11.1 ± 0.7 | 12.1 ± 0.08 |
| Vegan cookie | 0.16 ± 0.06 | 0.11 ± 0.03 | 1.31 ± 0.12 | 1.01 ± 0.01 |
| Normal cookie | 0.21 ± 0.08 | 0.33 ± 0.02 | 0.60 ± 0.16 | 0.55 ± 0.04 |
| Oat | 0.20 ± 0.11 | 0.19 ± 0.08 | 1.22 ± 0.07 | 1.12 ± 0.03 |
| Pineapple | 0.056 ± 0.030 | 0.055 ± 0.011 | 1.49 ± 0.16 | 1.13 ± 0.12 |
| Sesame | 0.24 ± 0.09 | 0.20 ± 0.03 | 1.24 ± 0.29 | 1.01 ± 0.05 |
| Hazelnut | 0.028 ± 0.030 | 0.029 ± 0.011 | 2.22 ± 0.16 | 2.04 ± 0.12 |
| Lupin | 0.19 ± 0.03 | 0.15 ± 0.02 | 1.34 ± 0.29 | 1.28 ± 0.09 |
| Soybean | 0.029 ± 0.024 | 0.025 ± 0.020 | 2.20 ± 0.21 | 2.01 ± 0.18 |
| Buckwheat flour | 0.25 ± 0.09 | 0.17 ± 0.01 | 1.34 ± 0.30 | 0.91 ± 0.10 |
| Allergen | Sensing Surface | Food Sample | Detection | LOD | Ref. | ||
|---|---|---|---|---|---|---|---|
| Transducer (Preparation Time) | Stability | Technique | Assay Time | ||||
| Ara h 1, Ara h 6 | Dual-SPCE (~12 h) | 30 days | Lupin, buckwheat flour, sesame, soybean, vegan cookies, raw peanut, healthy snack, peanut butter, peanut cookies | LSV (3-IP/Ag+) | 2 h 20 min | Ara h 1: 5.19 ng·mL−1; Ara h 6: 0.017 ng·mL−1 | This work |
| Ara h 1 | Silicon wafer/SWCNT(>15 h) | n.d. | n.d. | LSV (Label-free) | 30 min | 1 ng·mL−1 | [27] |
| SPCE/nAu (~12 h) | n.d. | Cookies, chocolate | LSV (3 IP/Ag+) | 3 h 50 min | 3.8 ng·mL−1 | [28] | |
| AuE/11-MUA (~19 h) | n.d. | n.d. | EIS (Fe(CN)63−/4−) | 5 min | 0.3 nM | [29] | |
| Ara h 6 | AuE/SWCNT (~14 h) | n.d. | Peanut butter, peanut pepper sauce, peanut chocolate, bean milk and chocolate milk | LSV (Label-free) | 30 min | 10 pg·L−1 | [30] |
| SPCE/nAu (~12 h) | n.d. | Cookies, chocolate | LSV (3-IP/Ag+) | 3 h 50 min | 0.27 ng·mL−1 | [31] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.; Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices. Foods 2021, 10, 1718. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10081718
Freitas M, Neves MMPS, Nouws HPA, Delerue-Matos C. Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices. Foods. 2021; 10(8):1718. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10081718
Chicago/Turabian StyleFreitas, Maria, Marta M. P. S. Neves, Henri P. A. Nouws, and Cristina Delerue-Matos. 2021. "Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices" Foods 10, no. 8: 1718. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10081718