Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SOB
2.3. Preparation of Ice Cream
2.4. Steady Shear Rheological Properties of Ice Cream Mixes
2.5. Particle Size of Ice Cream Mixes
2.6. Overrun of Ice Cream
2.7. Melting Properties of Ice Cream
2.8. Texture of Ice Cream
2.9. Physicochemical Properties of Ice Cream
2.10. Microstructure of Ice Cream
2.11. Sensory Evaluation of Ice Cream
2.12. Simulated Digestion of Ice Cream In Vitro
2.13. Determination of Free Fatty Acid
2.14. Determination of Protein Digestibility
2.15. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis
2.16. Statistical Analysis
3. Results
3.1. Apparent Viscosity of Ice Cream Mixes
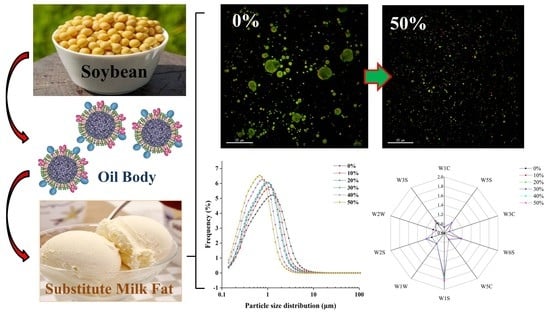
3.2. Particle Size of Ice Cream Mixes
3.3. Overrun of Ice Cream
3.4. Melting Properties of Ice Cream
3.5. Texture Properties of Ice Cream
3.6. Physicochemical Analysis of Ice Cream
3.7. Color Properties of Ice Cream
3.8. Microstructure of Ice Cream
3.9. Flavor Properties of Ice Cream
3.10. Sensory Score of Ice Cream
3.11. Particle Size of Ice Cream In Vitro Digestion
3.12. Free Fatty Acids of Ice Cream In Vitro Digestion
3.13. Protein Digestibility of Ice Cream In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurt, A.; Atalar, I. Effects of quince seed on the rheological, structural and sensory characteristics of ice cream. Food Hydrocoll. 2018, 82, 186–195. [Google Scholar] [CrossRef]
- Akbari, M.; Eskandari, M.H.; Davoudi, Z. Application and functions of fat replacers in low-fat ice cream: A review. Trends Food Sci. Technol. 2019, 86, 34–40. [Google Scholar] [CrossRef]
- Singh, H. Symposium review: Fat globules in milk and their structural modifications during gastrointestinal digestion. J. Dairy Sci. 2019, 102, 2749–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhao, P.; Li, J.; Wang, X.; Hou, J.; Jiang, Z. Effects of ultrasound synergized with microwave on structure and functional properties of transglutaminase-crosslinked whey protein isolate. Ultrason. Sonochem. 2022, 83, 105935. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Kelly, A. Physical chemistry of milk fat globules. In Advanced Dairy Chemistry, 3rd ed.; Fox, P., McSweeney, P., Eds.; Springer: New York, NY, USA, 2006; Volume 2, pp. 173–212. [Google Scholar]
- Wang, W.; Liu, F.; Xu, C.; Liu, Z.; Ma, J.; Gu, L.; Jiang, Z.; Hou, J. Lactobacillus plantarum 69-2 combined with galacto-oligosaccharides alleviates d-galactose-induced aging by regulating the AMPK/SIRT1 signaling pathway and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 2745–2757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022. [Google Scholar] [CrossRef]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhao, X.; Sun, R.; Sun, C.; Hou, D.; Zhang, X.; Jiang, L.; Hou, J.; Jiang, Z. Oil bodies extracted from high-oil soybeans (Glycine max) exhibited higher oxidative and physical stability than oil bodies from high-protein soybeans. Food Funct. 2022, 13, 3271–3282. [Google Scholar] [CrossRef]
- Zaaboul, F.; Zhao, Q.; Xu, Y.; Liu, Y. Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Wang, W.; Cui, C.; Wang, Q.; Sun, C.; Jiang, L.; Hou, J. Effect of pH on physicochemical properties of oil bodies from different oil crops. J. Food Sci. Technol. 2019, 56, 49–58. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Zhao, L.; Kong, X.; Hua, Y. Macronutrients and micronutrients of soybean oil bodies extracted at different pH. J. Food Sci. 2014, 79, C1285–C1291. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Matsakidou, A.; Kiosseoglou, V. Composition, properties and potential food applications of natural emulsions and cream materials based on oil bodies. RSC Adv. 2014, 4, 25067–25078. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Mu, S.; Ma, C.; Liu, Y.; Ma, Y.; Zhang, M.; Li, H.; Liu, X.; Hou, J.; Tian, B. Consequences of ball milling combined with high-pressure homogenization on structure, physicochemical and rheological properties of citrus fiber. Food Hydrocoll. 2022, 127, 107515. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Zhao, J.; Sun, R.; Shang, H.; Sun, C.; Liu, L.; Hou, J.; Jiang, Z. Physical and oxidative stability of astaxanthin microcapsules prepared with liposomes. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef] [PubMed]
- Pon, S.Y.; Lee, W.J.; Chong, G.h. Textural and rheological properties of stevia ice cream. Int. Food Res. J. 2015, 22, 1544–1549. [Google Scholar]
- AOAC International. Official method 2000.18. In Official Methods of Analysis of AOAC International, 21st ed.; Horwitz, W., Latimer, Jr., Eds.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Silva, J.M.; Klososki, S.J.; Silva, R.; Raices, R.S.L.; Silva, M.C.; Freitas, M.Q.; Barão, C.E.; Pimentel, T.C. Passion fruit-flavored ice cream processed with water-soluble extract of rice by-product: What is the impact of the addition of different prebiotic components? LWT 2020, 128, 109472. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z.; Wang, W.; Miao, Y.; Gu, L.; Li, Y.; Liu, X.; Jiang, L.; Hou, J.; Jiang, Z. NaCl induces flocculation and lipid oxidation of soybean oil body emulsions recovered by neutral aqueous extraction. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Yu, H.; Mu, S.; Li, H.; Liu, X.; Zhang, M.; Jiang, Z.; Hou, J. Biological activities and in vitro digestion characteristics of glycosylated α-lactalbumin prepared by microwave heating: Impacts of ultrasonication. LWT 2022, 158, 113141. [Google Scholar] [CrossRef]
- Hageman, J.H.J.; Keijer, J.; Dalsgaard, T.K.; Zeper, L.W.; Carrière, F.; Feitsma, A.L.; Nieuwenhuizen, A.G. Free fatty acid release from vegetable and bovine milk fat-based infant formulas and human milk during two-phase in vitro digestion. Food Funct. 2019, 10, 2102–2113. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, T.; Gantumur, M.-A.; Qayum, A.; Bilawal, A.; Jiang, Z.; Wang, L. Non-covalent interaction and digestive characteristics between α-lactalbumin and safflower yellow: Impacts of microwave heating temperature. LWT 2022, 159, 113206. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. Aqueous extraction of oil bodies from maize germ (Zea mays) and characterization of the resulting natural oil-in-water emulsion. J. Agric. Food Chem. 2009, 57, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Kayacier, A.; Toker, Ö.S.; Yilmaz, M.T.; Karaman, S. Steady, dynamic, creep, and recovery analysis of ice cream mixes added with different concentrations of xanthan gum. Food Bioproc. Tech. 2013, 6, 1420–1433. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Tehrani, M.M.; Mohebbi, M. Rheological and sensory properties of fat reduced vanilla ice creams containing milk protein concentrate (MPC). J. Food Meas. Charact. 2017, 11, 567–575. [Google Scholar] [CrossRef]
- Jirapeangtong, K.; Siriwatanayothin, S.; Chiewchan, N. Effects of coconut sugar and stabilizing agents on stability and apparent viscosity of high-fat coconut milk. J. Food Eng. 2008, 87, 422–427. [Google Scholar] [CrossRef]
- Bahramparvar, M.; Tehrani, M.M. Application and functions of stabilizers in ice cream. Food Rev. Int. 2011, 27, 389–407. [Google Scholar] [CrossRef]
- Soukoulis, C.; Tzia, C. Grape, raisin and sugarcane molasses as potential partial sucrose substitutes in chocolate ice cream: A feasibility study. Int. Dairy J. 2018, 76, 18–29. [Google Scholar] [CrossRef]
- Sun, C.; Wu, T.; Liu, R.; Liang, B.; Tian, Z.; Zhang, E.; Zhang, M. Effects of superfine grinding and microparticulation on the surface hydrophobicity of whey protein concentrate and its relation to emulsions stability. Food Hydrocoll. 2015, 51, 512–518. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Baba, A.S.; Misran, M. Effects of fermentation by Bifidobacterium bifidum on the rheology and physical properties of ice cream mixes made with cow and vegetable milks. Int. J. Food Sci. Technol. 2015, 50, 942–949. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Liu, Y.; Wu, T.; Zhang, M. Fabricating soy protein hydrolysate/xanthan gum as fat replacer in ice cream by combined enzymatic and heat-shearing treatment. Food Hydrocoll. 2018, 81, 39–47. [Google Scholar] [CrossRef]
- Muzammil, H.S.; Rasco, B.; Sablani, S. Effect of inulin and glycerol supplementation on physicochemical properties of probiotic frozen yogurt. Food Nutr. Res. 2017, 61, 1290314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, C.W.; Oh, N.S. Functional application of Maillard conjugate derived from a κ-carrageenan/milk protein isolate mixture as a stabilizer in ice cream. LWT 2022, 161, 113406. [Google Scholar] [CrossRef]
- Lomolino, G.; Zannoni, S.; Zabara, A.; Da Lio, M.; De Iseppi, A. Ice recrystallisation and melting in ice cream with different proteins levels and subjected to thermal fluctuation. Int. Dairy J. 2020, 100, 104557. [Google Scholar] [CrossRef]
- Chen, W.; Liang, G.; Li, X.; He, Z.; Zeng, M.; Gao, D.; Qin, F.; Goff, H.D.; Chen, J. Effects of soy proteins and hydrolysates on fat globule coalescence and meltdown properties of ice cream. Food Hydrocoll. 2019, 94, 279–286. [Google Scholar] [CrossRef]
- Javidi, F.; Razavi, S.M.A.; Behrouzian, F.; Alghooneh, A. The influence of basil seed gum, guar gum and their blend on the rheological, physical and sensory properties of low fat ice cream. Food Hydrocoll. 2016, 52, 625–633. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of instrumental texture properties from textural profile analysis (TPA) with eating behaviours and macronutrient composition for a wide range of solid foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Eskandari, M.H.; Niakosari, M.; Bedeltavana, A. The effect of inulin on the physicochemical properties and sensory attributes of low-fat ice cream. Int. Dairy J. 2016, 57, 52–55. [Google Scholar] [CrossRef]
- Kapchie, V.N.; Yao, L.; Hauck, C.C.; Wang, T.; Murphy, P.A. Oxidative stability of soybean oil in oleosomes as affected by pH and iron. Food Chem. 2013, 141, 2286–2293. [Google Scholar] [CrossRef]
- Speziali, G.; Liesinger, L.; Gindlhuber, J.; Leopold, C.; Pucher, B.; Brandi, J.; Castagna, A.; Tomin, T.; Krenn, P.; Thallinger, G.G.; et al. Myristic acid induces proteomic and secretomic changes associated with steatosis, cytoskeleton remodeling, endoplasmic reticulum stress, protein turnover and exosome release in HepG2 cells. J. Proteom. 2018, 181, 118–130. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals: A randomized controlled trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary bioactive fatty acids as modulators of immune function: Implications on human health. Nutrients 2019, 11, 2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenari, R.E.; Razavi, R. Effect of sonication conditions: Time, temperature and amplitude on physicochemical, textural and sensory properties of yoghurt. Int. J. Dairy Technol. 2021, 74, 332–343. [Google Scholar] [CrossRef]
- Çörekçi, B.; Toy, E.; ÖZTÜRK, F.; Malkoc, S.; Öztürk, B. Effects of contemporary orthodontic composites on tooth color following short-term fixed orthodontic treatment: A controlled clinical study. Turk. J. Med. Sci. 2015, 45, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Ding, W.; Ma, L.J.; Jia, R. Discrimination and characterization of different intensities of goaty flavor in goat milk by means of an electronic nose. J. Dairy Sci. 2015, 98, 55–67. [Google Scholar] [CrossRef]
- Silletti, E.; Vingerhoeds, M.H.; Norde, W.; van Aken, G.A. The role of electrostatics in saliva-induced emulsion flocculation. Food Hydrocoll. 2007, 21, 596–606. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, R.P.; Singh, H. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009, 23, 1563–1569. [Google Scholar] [CrossRef]
- Clulow, A.J.; Salim, M.; Hawley, A.; Boyd, B.J. A closer look at the behaviour of milk lipids during digestion. Chem. Phys. Lipids 2018, 211, 107–116. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhao, J.; Yu, R.; Hussain, M.A.; Qayum, A.; Jiang, Z.; Qu, B. Glycosylated whey protein isolate enhances digestion behaviors and stabilities of conjugated linoleic acid oil in water emulsions. Food Chem. 2022, 383, 132402. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, W.; He, R.; Xiong, F.; Ma, H. Protein breakdown and release of antioxidant peptides during simulated gastrointestinal digestion and the absorption by everted intestinal sac of rapeseed proteins. LWT 2017, 86, 424–429. [Google Scholar] [CrossRef]





| SOB Substitution Amounts (%) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Rheological | K (Pa·sn) | 0.22 ± 0.01 e | 0.32 ± 0.02 b | 0.26 ± 0.01 d | 0.31 ± 0.01 b | 0.36 ± 0.01 a | 0.29 ± 0.01 c |
| n | 0.65 ± 0.02 a | 0.56 ± 0.01 d | 0.61 ± 0.02 b | 0.58 ± 0.01 c | 0.54 ± 0.01 e | 0.59 ± 0.02 bc | |
| R2 | 0.9372 | 0.9396 | 0.9452 | 0.9403 | 0.941 | 0.9308 | |
| Particle size | D50 (μm) | 1.04 ± 0.02 a | 0.92 ± 0.02 b | 0.84 ± 0.01 c | 0.80 ± 0.01 d | 0.63 ± 0.01 e | 0.58 ± 0.01 f |
| D[4,3] (μm) | 1.36 ± 0.10 a | 1.15 ± 0.03 b | 1.02 ± 0.01 c | 0.97 ± 0.01 d | 0.77 ± 0.02 e | 0.69 ± 0.03 f | |
| D[3,2] (μm) | 0.72 ± 0.01 a | 0.67 ± 0.03 b | 0.63 ± 0.01 c | 0.60 ± 0.01 d | 0.50 ± 0.01 e | 0.46 ± 0.01 f | |
| PDI | 0.84 ± 0.03 a | 0.68 ± 0.01 b | 0.64 ± 0.00 c | 0.61 ± 0.01 d | 0.58 ± 0.01 e | 0.53 ± 0.02 f | |
| SOB Substitution Amounts (%) | ||||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | |
| Hardness (g) | 5899.93 ± 56.69 a | 5506.79 ± 92.58 b | 5042.31 ± 130.08 c | 4740.50 ± 96.28 d | 4326.72 ± 79.66 e | 4223.43 ± 47.14 f |
| Adhesiveness (g·s) | 138.97 ± 2.80 f | 165.94 ± 4.59 e | 221.68 ± 1.78 d | 256.21 ± 6.72 c | 270.37 ± 9.10 b | 315.96 ± 4.35 a |
| Springiness | 0.73 ± 0.02 d | 0.78 ± 0.01 c | 0.80 ± 0.01 bc | 0.81 ± 0.01 b | 0.82 ± 0.01 ab | 0.84 ± 0.04 a |
| Chewiness | 243.18 ± 5.15 e | 263.56 ± 10.98 d | 314.82 ± 4.80 c | 346.46 ± 6.96 b | 384.99 ± 4.10 a | 392.91 ± 2.14 a |
| SOB Substitution Amounts (%) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| Physicochemical properties | TSS °Bx | 30.65 ± 0.03 f | 30.81 ± 0.04 e | 30.87 ± 0.02 d | 30.97 ± 0.03 c | 31.04 ± 0.02 b | 31.08 ± 0.01 a |
| Fat % | 5.97 ± 0.04 a | 5.88 ± 0.03 b | 5.78 ± 0.04 c | 5.71 ± 0.05 d | 5.63 ± 0.02 e | 5.53 ± 0.03 f | |
| Protein % | 5.57 ± 0.03 f | 5.68 ± 0.04 e | 5.76 ± 0.03 d | 5.84 ± 0.05 c | 5.92 ± 0.04 b | 6.01 ± 0.02 a | |
| Carbohydrate % | 18.14 ± 0.06 f | 18.25 ± 0.05 e | 18.34 ± 0.06 d | 18.42 ± 0.03 c | 18.48 ± 0.02 b | 18.53 ± 0.04 a | |
| Fatty acid content | Myristic acid % | 5.92 ± 0.01 a | 5.34 ± 0.03 b | 4.96 ± 0.08 c | 4.51 ± 0.10 d | 4.31 ± 0.04 e | 3.79 ± 0.03 f |
| Hyperic acid % | 27.25 ± 0.11 a | 26.40 ± 0.03 b | 25.61 ± 0.02 c | 24.25 ± 0.60 d | 23.93 ± 0.22 e | 22.54 ± 0.17 f | |
| Glycolic acid % | 9.08 ± 0.08 a | 8.64 ± 0.00 b | 8.36 ± 0.10 b | 8.06 ± 0.19 c | 7.95 ± 0.18 c | 7.40 ± 0.04 d | |
| Oleic acid % | 25.65 ± 0.06 a | 25.90 ± 0.01 a | 25.93 ± 0.32 a | 25.98 ± 0.51 a | 26.10 ± 0.33 a | 26.20 ± 0.08 a | |
| Linoleic acid % | 4.78 ± 0.03 f | 7.90 ± 0.04 e | 11.13 ± 0.12 d | 14.48 ± 0.20 c | 15.97 ± 0.01 b | 17.95 ± 0.13 a | |
| Linolenic acid % | 0.34 ± 0.01 f | 0.96 ± 0.03 e | 1.40 ± 0.04 d | 1.85 ± 0.01 c | 2.05 ± 0.02 b | 2.31 ± 0.01 a | |
| Color properties | L* | 95.95 ± 2.10 a | 97.40 ± 0.59 a | 96.60 ± 0.56 a | 96.86 ± 2.02 a | 97.90 ± 1.31 a | 98.83 ± 3.87 a |
| a* | 0.64 ± 0.02 a | 0.56 ± 0.02 b | 0.41 ± 0.05 c | 0.45 ± 0.03 c | 0.35 ± 0.03 d | 0.30 ± 0.02 e | |
| b* | 21.31 ± 0.27 ab | 20.65 ± 0.05 b | 21.42 ± 0.08 a | 20.97 ± 0.17 ab | 21.32 ± 0.33 ab | 21.52 ± 0.79 a | |
| ΔE | - | 1.60 | 0.70 | 0.99 | 1.97 | 2.91 | |
| SOB Substitution Amounts (%) | Color | Taste | Texture | Flavor | Sensory Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | G | M | P | E | G | M | P | E | G | M | P | E | G | M | P | ||
| 0 | 7 | 3 | 0 | 0 | 5 | 3 | 2 | 0 | 6 | 3 | 1 | 0 | 7 | 3 | 0 | 0 | 8.06 ± 0.19 bc |
| 10 | 7 | 3 | 0 | 0 | 2 | 5 | 2 | 1 | 4 | 4 | 1 | 1 | 6 | 3 | 1 | 0 | 7.44 ± 0.19 d |
| 20 | 7 | 3 | 0 | 0 | 3 | 5 | 2 | 0 | 5 | 4 | 1 | 0 | 8 | 1 | 1 | 0 | 7.89 ± 0.34 c |
| 30 | 8 | 2 | 0 | 0 | 3 | 6 | 1 | 0 | 8 | 2 | 0 | 0 | 8 | 2 | 0 | 0 | 8.26 ± 0.24 ab |
| 40 | 9 | 1 | 0 | 0 | 5 | 4 | 1 | 0 | 8 | 2 | 0 | 0 | 9 | 1 | 0 | 0 | 8.46 ± 0.20 a |
| 50 | 8 | 2 | 0 | 0 | 5 | 5 | 0 | 0 | 8 | 2 | 0 | 0 | 7 | 3 | 0 | 0 | 8.38 ± 0.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties. Foods 2022, 11, 1504. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101504
Wang W, Wang M, Xu C, Liu Z, Gu L, Ma J, Jiang L, Jiang Z, Hou J. Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties. Foods. 2022; 11(10):1504. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101504
Chicago/Turabian StyleWang, Wan, Min Wang, Cong Xu, Zhijing Liu, Liya Gu, Jiage Ma, Lianzhou Jiang, Zhanmei Jiang, and Juncai Hou. 2022. "Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties" Foods 11, no. 10: 1504. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101504