Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Treatment
2.2. EPE Method
2.3. CPE Method
2.4. UAE Method
2.5. SFE Method
2.6. Quality Assessment
2.7. Oxidative Stability Using the Rancimat Method
2.8. Color Measurement
2.9. Determination of Plant Pigment Content
2.10. Determination of Total Polyphenol Content
2.11. DPPH Radical Scavenging Activity
2.12. ABTS Radical Scavenging Activity
2.13. Statistical Analyses
3. Results and Discussion
3.1. Quality Assessment of Oil
3.2. Oxidative Stability of Sinami Oil
3.3. Color Properties of Oil
3.4. Plant Pigments, Total Polyphenols and Antioxidant Activity of Sinami Oil
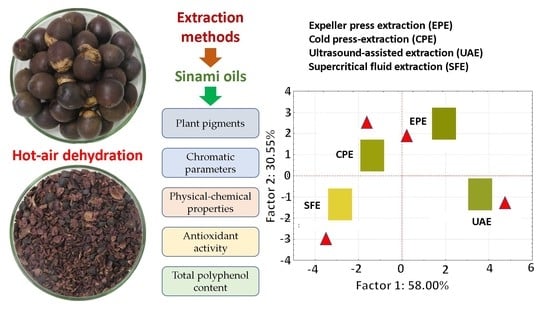
3.5. PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balslev, H.; Grandez, C.; Paniagua Zambrana, N.Y.; Møller, A.L.; Hansen, S.L. Palmas (Arecaceae) útiles en los alrededores de Iquitos, Amazonía Peruana. Rev. Peru. Biol. 2008, 15 (Suppl. 1), 121–132. [Google Scholar] [CrossRef] [Green Version]
- Navas Hernández, P.B.; Fregapane, G.; Salvador, M.D. Bioactive compounds, volatiles, and antioxidant activity of virgin seje oils (Jessenia bataua) from the Amazonas. J. Food Lipids 2009, 16, 629–644. [Google Scholar] [CrossRef]
- Ocampo-Duran, A.; Fernández-Lavado, A.P.; Castro-Lima, F. Aceite de la palma de seje Oenocarpus bataua Mart. por su calidad nutricional puede contribuir a la conservación y uso sostenible de los bosques de galería en la Orinoquia Colombiana. Orinoquia 2013, 17, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.H.H.; Sena, C.; Santos, O.V.; da Costa, W.A.; Rodrigues, A.M.C.; Carvalho Junior, R.N. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas Aceites 2018, 69, e246. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.C.; Lacerda, E.C.Q.; Santos, L.S.; Ferrão, S.P.B.; Fontan, R.C.I.; Veloso, C.M.; Bonomo, R.C.F. Antioxidant activity and bioactive compounds of babassu (Orbignya phalerata) virgin oil obtained by different methods of extraction. Open Food Sci. J. 2019, 11, 35–43. [Google Scholar] [CrossRef]
- Barroso, M.E.S.; Oliveira, B.G.; Pimentel, E.F.; Pereira, P.M.; Ruas, F.G.; Andrade, T.U.; Lenz, D.; Scherer, R.; Fronza, M.; Ventura, J.A.; et al. Phytochemical profile of genotypes of Euterpe edulis Martius—Juçara palm fruits. Int. Food Res. J. 2019, 116, 985–993. [Google Scholar] [CrossRef]
- Best, I.; Rengifo, H.; Velarde, E.; Loja, J.F.; Portugal, A.; Rengifo, P.; Aguilar, L.; Ramos-Escudero, F.; Muñoz, A.M. Phenology of Oenocarpus mapora H. Karst in low-terrace and high-terrace forests of the Madre de Dios Region, Peru. Forests 2021, 12, 1424. [Google Scholar] [CrossRef]
- De Souza, F.G.; Náthia-Neves, G.; de Araújo, F.F.; Dias Audibert, F.L.; Delafiori, J.; Neri-Numa, I.A.; Catharino, R.R.; de Alencar, S.M.; de Almeida Meireles, M.A.; Pastore, G.M. Evaluation of antioxidant capacity, fatty acid profile, and bioactive compounds from buritirana (Mauritiella armata Mart.) oil: A little-explored native Brazilian fruit. Food Res. Int. 2021, 142, 110260. [Google Scholar] [CrossRef]
- Sosnowska, J.; Walanus, A.; Balslev, H. Asháninka palm management and domestication in the Peruvian amazon. Hum. Ecol. Interdiscip. J. 2015, 43, 451–466. [Google Scholar] [CrossRef] [Green Version]
- Quiñones Ruiz, C.E. Determinación de Polifenoles Totales, Antocianinas y Capacidad Antioxidante del Ungurahui (Oenocarpus bataua Mart.), Sinamillo (Oenocarpus mapora H. Karst.) y Huasai (Euterpe oleracea Mart.). Master’s Thesis, Universidad Nacional Agraria de la Selva, Tingo María, Peru, 2018. [Google Scholar]
- Rezaire, A.; Robinson, J.-C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Durand, P.; Prost, E.; Fils-Lycaon, B. Amazonian palm Oenocarpus bataua (“patawa”): Chemical and biological antioxidant activity—Phytochemical composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef]
- Carvalho, A.V.; da Silveira, T.F.; de Sousa, S.H.B.; de Moraes, M.R.; Godoy, H.T. Phenolic composition, and antioxidant capacity of bacaba-de-leque (Oenocarpus distichus Mart.) genotypes. J. Food Compost. Anal. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- De Sousa, S.H.B.; Mattietto, R.A.; Chisté, R.C.; Carvalho, A.V. Phenolic compounds are highly correlated to the antioxidant capacity of genotypes of Oenocarpus distichus Mart fruits. Food Res. Int. 2018, 108, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cól, C.D.; Tischer, B.; Flôres, S.H.; Rech, R. Foam-mat drying of bacaba (Oenocarpus bacaba): Process characterization, physicochemical properties, and antioxidant activity. Food Bioprod. Process. 2021, 126, 23–31. [Google Scholar] [CrossRef]
- Szyczewski, P.; Frankowski, M.; Zioła-Frankowska, A.; Siepak, J.; Szyczewski, T.; Piotrowski, P. A comparative study of the content of heavy metals in oils: Linseed oil, rapeseed oil and soybean oil in technological production processes. Arch. Environ. Prot. 2016, 42, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible plant oil: Global status, health issues, and perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Commission Regulation (EEC). No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991R2568-20191020 (accessed on 1 February 2022).
- Endo, Y. Analytical methods to evaluate the quality of edible fats and oils: The JOCS standard methods for analysis of fats, oils and related materials (2013) and advanced methods. J. Oleo Sci. 2018, 67, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Negash, Y.A.; Amare, D.E.; Bitew, B.D.; Dagne, H. Assessment of quality of edible vegetable oils accessed in Gondar City, Northwest Ethiopia. BMC Res. Notes 2018, 12, 793. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [Green Version]
- Martínez Nieto, L.; Hodaifa, G.; Lozano Peña, J.L. Changes in phenolic compounds and Rancimat stability of olive oils from varieties of olives at different stages of ripeness. J. Sci. Food Agric. 2010, 90, 2393–2398. [Google Scholar] [CrossRef]
- Salta, F.N.; Mylona, A.; Chiou, A.; Boskou, G.; Andrikopoulos, N.K. Oxidative stability of edible vegetable oils enriched in polyphenols with olive leaf extract. Food Sci. Technol. Int. 2007, 13, 413–421. [Google Scholar] [CrossRef]
- Dhavamani, S.; Poorna Chandra Rao, Y.; Lokesh, B.R. Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo: A photochemiluminescence based analysis. Food Chem. 2014, 164, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Escudero, F.; Morales, M.T.; Asuero, A.G. Characterization of bioactive compounds from monovarietal virgin olive oils: Relationship between phenolic compounds-antioxidant capacities. Int. J. Food Prop. 2015, 18, 348–358. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.; Gülmez, Ö.; Kurtulbaş, E.; Yazar, S. Improving the quality of vegetable oils treated with phytochemicals: A comparative study. J. Food Sci. Technol. 2020, 57, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Cicero, N.; Albergamo, A.; Salvo, A.; Bua, G.D.; Bartolomeo, G.; Mangano, V.; Rotondo, A.; Di Stefano, V.; Di Bella, G.; Dugo, G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Res. Int. 2018, 109, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sey, A.A.; Pham, T.H.; Kavanagh, V.; Kaur, S.; Cheema, M.; Galagedara, L.; Thomas, R. Canola produced under boreal climatic conditions in Newfoundland and Labrador have a unique lipid composition and expeller press extraction retained the composition for commercial use. J. Adv. Res. 2020, 24, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Introduction to cold pressed oils: Green technology, bioactive compounds, functionality, and applications. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Ramadan, M.F., Ed.; Elsevier Inc.: London, UK, 2020; pp. 1–5. [Google Scholar]
- Dong, W.; Chen, Q.; Wei, C.; Hu, R.; Long, Y.; Zong, Y.; Chu, Z. Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason. Sonochem. 2021, 74, 105578. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Yan, Y.-Y.; Liu, H.-M.; Qi, K.; Zhu, X.-L.; Wang, X.-D.; Qin, G.-Y. Subcritical low temperature extraction technology and its application in extracting seed oils. J. Food Process Eng. 2021, 44, e13805. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Escudero, F.; Morales, M.T.; Ramos Escudero, M.; Muñoz, A.M.; Cancino Chavez, K.; Asuero, A.G. Assessment of phenolic and volatile compounds of commercial Sacha inchi oils and sensory evaluation. Food Res. Int. 2021, 140, 110022. [Google Scholar] [CrossRef]
- Žanetić, M.; Špika, M.J.; Ožić, M.M.; Bubola, K.B. Comparative study of volatile compounds and sensory characteristics of dalmatian monovarietal virgin olive oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; da Silva, M.P.; de Sousa, S.H.B.; Bezerra, P.N.; Menezes, E.G.O.; da Silva, N.J.N.; Banna, D.A.D.S.; Araújo, M.E.; Junior, R.N.C. (2019). Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Zahir, E.; Saeed, R.; Hameed, M.A.; Yousuf, A. Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier transform-infrared (FT-IR) spectroscopy. Arab. J. Chem. 2017, 10, S3870–S3876. [Google Scholar] [CrossRef] [Green Version]
- International Olive Council (IOC). International Olive Council IOC/T20/Doc. No. 19/Rev3 2010. Method of Analysis: Spectrophotometric Investigation in the Ultraviolet. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 6 February 2022).
- American Oil Chemists’ Society (AOCS). Official Method Cd 18-90. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Urbana, IL, USA, 2017. [Google Scholar]
- Heller, M.; Gemming, L.; Tung, C.; Grant, R. Oxidation of fish oil supplements in Australia. Int. J. Food Sci. Nutr. 2019, 70, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zheng, L.; Jin, J.; Li, X.; Fu, J.; Wang, M.; Guan, Y.; Song, X. Phytochemical, and biological characteristics of Mexican chia seed oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Escudero, F.; González-Miret, M.L.; Viñas-Ospino, A.; Ramos-Escudero, M. Quality, stability, carotenoids, and chromatic parameters of commercial Sacha inchi oil originating from Peruvian cultivars. J. Food Sci. Technol. 2019, 56, 4901–4910. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Gómez-Coca, R.B.; Muñoz, A.M.; De La Fuente-Carmelino, L.; Pérez-Camino, M.C. Oil from three aguaje morphotypes (Mauritia flexuosa L.f.) extracted by supercritical fluid with CO2: Chemical composition and chromatic properties. Front. Sustain. Food Syst. 2022, 6, 843772. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Ribeiro, D.N.; Alves, F.M.S.; Ramos, V.H.R.; Alves, P.; Narain, N.; Vedoy, D.R.L.; Cardozo-Filho, L.; de Jesus, E. Extraction of passion fruit (Passiflora cincinnata Mast.) pulp oil using pressurized ethanol and ultrasound: Antioxidant activity and kinetics. J. Supercrit. Fluids 2020, 165, 104944. [Google Scholar] [CrossRef]
- Rojo-Gutiérrez, E.; Carrasco-Molinar, O.; Tirado-Gallegos, J.M.; Levario-Gómez, A.; Chávez-González, M.L.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J. Evaluation of green extraction processes, lipid composition and antioxidant activity of pomegranate seed oil. J. Food Meas. Charact. 2021, 15, 2098–2107. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative methods of bioactive compounds and oils extraction from berry fruit by-products—A review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.K. A review on application of ultrasound and ultrasound assisted technology for seed oil extraction. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.E.A.; Ma, M.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.S.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, E. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurement and modeling. Int. J. Food Prop. 2017, 20, S1965–S1981. [Google Scholar] [CrossRef] [Green Version]
- Ceriani, R.; Paiva, F.R.; Gonçalves, C.B.; Batista, E.A.C.; Meirelles, A.J.A. Densities and viscosities of vegetable oils of nutritional value. J. Chem. Eng. Data. 2008, 53, 1846–1853. [Google Scholar] [CrossRef]
- Yunus, W.M.M.; Fen, Y.W.; Yee, L.M. Refractive index and Fourier transform infrared spectra of virgin coconut oil and virgin olive oil. Am. J. Appl. Sci. 2009, 6, 328–331. [Google Scholar] [CrossRef]
- Serra, J.L.; Rodrigues, A.M.C.; Rilton, A.F.; Meirelles, A.J.A.; Darnet, S.H.; da Silva, L.H.M. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef]
- Aparicio, R.; Conte, L.S.; Fiebig, H.-J. Olive oil authentication. In Handbook of Olive Oil: Analysis and Properties, 2nd ed.; Aparicio, R., Harwood, J., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; Chapter 16; pp. 589–653. [Google Scholar]
- Morales, M.T.; Przybylski, R. Olive oil oxidation. In Handbook of Olive Oil: Analysis and Properties, 2nd ed.; Aparicio, R., Harwood, J., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; Chapter 13; pp. 479–522. [Google Scholar]
- Karoui, R. Food authenticity and fraud. In Chemical Analysis of Food: Techniques and Applications, 2nd ed.; Pico, Y., Ed.; Elsevier Inc.: London, UK, 2020; Chapter 13; pp. 579–608. [Google Scholar]
- Shi, L.-K.; Zheng, L.; Liu, R.-J.; Chang, M.; Jin, Q.-Z.; Wang, X.-G. Chemical characterization, oxidative stability, a nd in vitro antioxidant capacity of sesame oils extracted by supercritical a nd subcritical techniques and conventional methods: A comparative study using chemometrics. Eur. J. Lipid Sci. Technol. 2018, 120, 1700326. [Google Scholar] [CrossRef]
- Korifi, R.; Plard, J.; Le Dréau, Y.; Rébufa, C.; Rutledge, D.N.; Dupuy, N. Highlighting metabolic indicators of olive oil during storage by the AComDim method. Food Chem. 2016, 203, 104–116. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Emerging new energy crops for biodiesel production. In Energy, Biodiesel Science and Technology, Bart, J.C.J., Palmeri, N., Cavallaro, S., Eds.; Woodhead Publishing: Cambridge, UK, 2010; Chapter 6; pp. 226–284. [Google Scholar]
- Cheikhyoussef, N.; Kandawa-Schulz, M.; Böck, R.; Cheikhyoussef, A. Cold pressed Moringa oleifera seed oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Ramadan, M.F., Ed.; Elsevier Inc.: London, UK, 2020; Chapter 42; pp. 467–475. [Google Scholar]
- Tilahun, W.W.; Grossi, J.A.S.; Favaro, S.P.; Sediyama, C.S.; Goulart, S.D.M.; Pimentel, L.D.; Motoike, S.Y. Increase in oil content and changes in quality of macauba mesocarp oil along storage. Oilseeds Fats Crops Lipids 2019, 26, 20. [Google Scholar] [CrossRef] [Green Version]
- Santos, O.V.; Gonçalves, B.S.; Macedo, C.S.; Conceição, L.R.V.; Costa, C.E.F.; Monteiro Júnior, O.V.; Souza, A.L.G.; Lannes, S.C.S. Evaluation of quality parameters and chromatographic, spectroscopic, and thermogravimetric profile of Patauá oil (Oenocarpus bataua). Food Sci. Technol. 2020, 40, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.M.; Sampaio, K.A.; Taham, T.; Rocco, S.A.; Ceriani, R.; Meirelles, A.J.A. Characterization of oil extracted from buriti fruit (Mauritia flexuosa) grown in the Brazilian amazon region. J. Am. Oil Chem. Soc. 2009, 86, 611–616. [Google Scholar] [CrossRef]
- Rigane, G.; Yahyaoui, A.; Acar, A.; Mnif, S.; Salem, R.B.; Arslan, D. Change in some quality parameters and oxidative stability of olive oils with regard to ultrasound pretreatment, depitting and water addition. Biotechnol. Rep. 2020, 26, e00442. [Google Scholar] [CrossRef] [PubMed]
- Halim, H.H.; Thoo, Y.Y. Effect of ultrasound treatment on oxidative stability of sunflower oil and palm oil. Int. Food Res. J. 2018, 25, 1959–1967. [Google Scholar]
- Belayneh, H.D.; Wehling, R.L.; Cahoon, E.B.; Ciftci, O.N. Effect of extraction method on the oxidative stability of camelina seed oil studied by differential scanning calorimetry. J. Food Sci. 2017, 82, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, J.; Ortiz-Viedma, J. Edible oil parameters during deterioration processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (II): CIELUV and CIELAB uniform colour spaces. Food Res. Int. 2008, 41, 513–521. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Dong, B.; Huang, Y.; Bao, Z.; Zhao, H. Relationship between pigment composition and peel color for the fruit of Chinese flame tree. J. Am. Soc. Hortic. Sci. 2018, 143, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Best, I.; Casimiro-Gonzales, S.; Portugal, A.; Olivera-Montenegro, L.; Aguilar, L.; Muñoz, A.M.; Ramos-Escudero, F. Phytochemical screening and DPPH radical scavenging activity of three morphotypes of Mauritia flexuosa L.f. from Peru, and thermal stability of a milk-based beverage enriched with carotenoids from these fruits. Heliyon 2020, 6, e05209. [Google Scholar] [CrossRef]
- Kılıç, K.; Onal-Ulusoy, B.; Boyacı, İ.H. A novel method for color determination of edible oils in L*a*b* format. Eur. J. Lipid Sci. Technol. 2007, 109, 157–164. [Google Scholar] [CrossRef]
- Paciulli, M.; Difonzo, G.; Conte, P.; Flamminii, F.; Piscopo, A.; Chiavaro, E. Physical and thermal evaluation of olive oils from minor Italian cultivars. Foods 2021, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Nobre, B.P.; Mendes, R.L.; Queiroz, E.M.; Pessoa, F.L.P.; Coelho, J.P.; Palavra, F. Supercritical carbon dioxide extraction of pigments from Bixa orellana seeds (experiments and modeling). Braz. J. Chem. Eng. 2006, 23, 251–258. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-solvent selection for supercritical fluid extraction of astaxanthin and other carotenoids from Penaeus monodon waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Konuşkan, D.B. Minor bioactive lipids in cold pressed oils. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Ramadan, M.F., Ed.; Elsevier Inc.: London, UK, 2020; Chapter 2; pp. 7–14. [Google Scholar]
- Lara-Abia, S.; Welti-Chanes, J.; Cano, M.P. Effect of ultrasound-assisted extraction of carotenoids from papaya (Carica papaya L. cv. Sweet Mary) using vegetable oils. Molecules 2022, 27, 638. [Google Scholar] [CrossRef]
- Murillo Pulgarín, J.A.; García Bermejo, L.F.; Carrasquero Durán, A. Evaluation of the antioxidant activity of vegetable oils based on luminol chemiluminescence in a microemulsion. Eur. J. Lipid Sci. Technol. 2010, 112, 1294–1301. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef] [Green Version]




| Parameters | CPE | EPE | UAE | SFE |
|---|---|---|---|---|
| Extraction Yield (%) | 5.52 | 6.99 | 15.64 | 7.45 |
| Density (g/mL) | 0.95 ± 0.01 a | 0.89 ± 0.02 b | 0.89 ± 0.01 b | 0.94 ± 0.01 ab |
| Refractive Index (20 °C) | 1.4690 a | 1.4691 a | 1.4650 b | 1.4690 a |
| Specific Extinction (K232) | 3.10 ± 0.05 b | 3.10 ± 0.08 b | 3.93 ± 0.13 a | 3.19 ± 0.11 b |
| Specific Extinction (K270) | 0.35 ± 0.01 c | 0.58 ± 0.03 b | 0.77 ± 0.03 a | 0.54 ± 0.02 b |
| Extinction Coefficient Variation (∆K) | 0.006 d | 0.018 b | 0.017 c | 0.018 a |
| Free Acidity (%) | 0.56 ± 0.09 b | 0.55 ± 0.09 b | 1.05 ± 0.08 a | 1.05 ± 0.08 a |
| Peroxide Value (meq O2/kg) | 22.76 ± 0.07 c | 22.66 ± 0.13 c | 43.58 ± 0.27 a | 25.88 ± 0.17 b |
| pAV | 4.06 ± 0.12 a | 4.13 ± 0.06 a | 3.96 ± 0.04 a | 2.11 ± 0.11 b |
| TOTOX | 53.65 ± 0.37 c | 53.58 ± 0.15 c | 95.08 ± 0.62 a | 55.97 ± 0.56 b |
| Saponification Value (mg KOH/g) | 202.17 ± 1.91 b | 202.75 ± 0.85 b | 196.08 ± 1.82 c | 219.75 ± 0.64 a |
| Oils | Extraction System | Stability Parameter | Reference | ||
|---|---|---|---|---|---|
| T (°C) | Flow Rate (L/h) | OSI (h) | |||
| Sinami | CPE | 110 | 20 | 2.69 ± 0.17 b | |
| Sinami | EPE | 110 | 20 | 5.53 ± 0.46 a | |
| Sinami | UAE | 110 | 20 | 1.99 ± 0.32 b | |
| Sinami | SFE | 110 | 20 | 5.01 ± 0.08 a | |
| Buriti (blend) | NS | 110 | 9 | 18.30 | [62] |
| Seje | CPE | 120 | 20 | 4.28 | [2] |
| Macaúba | NS | 110 | 10 | 16.0 | [60] |
| Bacaba | SFE | 110 | 10 | 5.39 | [4] |
| Patawa | CPE | 100 | 10 | 2.79 | [61] |
| Extraction System | Input Color Value | L* | a* | b* | C*ab | hab | View |
|---|---|---|---|---|---|---|---|
| CPE | rgb 139 149 3 | 58.96 | −18.73 | 61.88 | 64.65 | 73.16 |  |
| rgb 146 148 7 | 59.43 | −15.21 | 61.78 | 63.63 | 76.16 | ||
| rgb 144 149 7 | 59.48 | −16.76 | 61.84 | 64.07 | 74.84 | ||
| rgb 158 162 14 | 64.23 | −16.90 | 64.83 | 66.99 | 75.39 | ||
| rgb 161 165 15 | 65.34 | −17.27 | 65.60 | 67.83 | 75.25 | ||
| rgb 160 164 18 | 64.90 | −17.07 | 64.77 | 66.98 | 75.26 | ||
| rgb 165 169 16 | 66.95 | −17.54 | 66.99 | 69.25 | 75.32 | ||
| rgb 162 167 15 | 66.04 | −17.62 | 66.23 | 68.53 | 75.10 | ||
| rgb 171 173 17 | 68.42 | −16.63 | 68.26 | 70.25 | 76.30 | ||
| rgb 140 149 3 | 59.02 | −18.29 | 61.91 | 64.56 | 73.55 | ||
| EPE | rgb 137 143 4 | 56.97 | −16.37 | 60.06 | 62.26 | 74.75 |  |
| rgb 151 158 7 | 62.54 | −17.99 | 64.37 | 66.83 | 74.38 | ||
| rgb 139 145 4 | 57.78 | −16.77 | 60.74 | 63.02 | 74.57 | ||
| rgb 138 141 6 | 56.45 | −15.24 | 59.42 | 61.35 | 75.61 | ||
| rgb 133 137 4 | 54.90 | −15.21 | 58.44 | 60.39 | 75.41 | ||
| rgb 153 158 7 | 62.65 | −17.25 | 64.51 | 66.78 | 75.03 | ||
| rgb 143 150 2 | 59.43 | −17.58 | 62.48 | 64.90 | 74.29 | ||
| rgb 130 137 2 | 54.61 | −16.41 | 58.35 | 60.61 | 74.29 | ||
| rgb 131 139 1 | 55.41 | −17.46 | 59.21 | 61.73 | 73.57 | ||
| rgb 132 140 1 | 55.78 | −17.71 | 59.49 | 62.07 | 73.42 | ||
| UAE | rgb 155 167 51 | 65.51 | −19.06 | 55.37 | 58.56 | 71.00 |  |
| rgb 144 154 41 | 60.87 | −18.15 | 54.56 | 57.50 | 71.60 | ||
| rgb 146 155 38 | 61.52 | −18.12 | 56.27 | 59.11 | 72.15 | ||
| rgb 152 165 38 | 64.67 | −20.17 | 59.13 | 62.48 | 71.17 | ||
| rgb 147 159 33 | 62.50 | −19.38 | 58.78 | 61.89 | 71.75 | ||
| rgb 148 161 31 | 63.39 | −20.38 | 59.96 | 63.32 | 71.23 | ||
| rgb 149 161 31 | 63.20 | −19.89 | 59.97 | 63.18 | 71.65 | ||
| rgb 152 164 38 | 64.39 | −19.96 | 59.08 | 62.36 | 71.33 | ||
| rgb 154 165 48 | 65.04 | −19.26 | 56.20 | 59.41 | 71.09 | ||
| rgb 144 154 42 | 60.90 | −17.98 | 54.04 | 56.95 | 71.60 | ||
| SFE | rgb 168 174 135 | 83.48 | −10.23 | 73.96 | 74.67 | 82.12 |  |
| rgb 163 170 129 | 82.12 | −9.99 | 72.38 | 73.05 | 82.19 | ||
| rgb 156 164 122 | 83.05 | −10.01 | 73.45 | 74.12 | 82.24 | ||
| rgb 171 177 131 | 82.31 | −10.04 | 72.36 | 73.05 | 82.10 | ||
| rgb 167 174 128 | 83.64 | −10.19 | 74.66 | 75.36 | 82.23 | ||
| rgb 165 173 124 | 82.15 | −10.11 | 72.38 | 73.08 | 82.05 | ||
| rgb 175 180 131 | 83.10 | −10.09 | 73.59 | 74.28 | 82.19 | ||
| rgb 166 173 122 | 81.97 | −10.09 | 72.57 | 73.17 | 82.09 | ||
| rgb 169 175 124 | 82.14 | −9.81 | 72.13 | 72.80 | 82.26 | ||
| rgb 161 168 116 | 83.06 | −10.10 | 74.16 | 74.85 | 82.24 | ||
| CPE | 63.28 ± 3.67 b | −17.20 ± 0.97 b | 64.41 ± 2.42 b | 66.67 ± 2.34 b | 75.0.3 ± 1.00 b | ||
| EPE | 57.65 ± 2.97 c | −16.80 ± 0.99 b | 60.71 ± 2.30 c | 62.99 ± 2.38 c | 74.53 ± 0.71 b | ||
| UAE | 63.20 ± 1.71 b | −19.23 ± 0.89 a | 57.34 ± 2.28 d | 60.48 ± 2.42 d | 71.46 ± 0.36 c | ||
| SFE | 82.70 ± 0.63 a | −10.06 ± 0.12 c | 73.16 ± 0.91 a | 73.05 ± 0.91 a | 82.17 ± 0.07 a | ||
| Extraction System | Plant Pigments | Total Phenolics | DPPH (IC50), mg/mL | ABTS (IC50), mg/mL | |
|---|---|---|---|---|---|
| Carotenoids | Chlorophylls | ||||
| CPE | 47.13 ± 0.02 b | 56.24 ± 0.10 b | 89.03 ± 5.87 d | 5.70 ± 0.32 c | 13.12 ± 0.10 c |
| EPE | 51.62 ± 0.65 a | 109.56 ± 1.14 a | 116.72 ± 1.96 c | 3.77 ± 0.37 ab | 11.86 ± 0.02 b |
| UAE | 42.32 ± 0.04 c | 111.67 ± 0.68 a | 615.18 ± 3.92 a | 3.24 ± 0.33 a | 7.81 ± 0.43 a |
| SFE | 12.29 ± 0.29 d | 3.48 ± 1.25 c | 200.49 ± 4.90 b | 4.58 ± 0.21 b | 11.28 ± 0.11 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.M.; Casimiro-Gonzales, S.; Gómez-Coca, R.B.; Moreda, W.; Best, I.; Cajo-Pinche, M.I.; Loja, J.F.; Ibañez, E.; Cifuentes, A.; Ramos-Escudero, F. Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity. Foods 2022, 11, 1518. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101518
Muñoz AM, Casimiro-Gonzales S, Gómez-Coca RB, Moreda W, Best I, Cajo-Pinche MI, Loja JF, Ibañez E, Cifuentes A, Ramos-Escudero F. Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity. Foods. 2022; 11(10):1518. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101518
Chicago/Turabian StyleMuñoz, Ana María, Sandra Casimiro-Gonzales, Raquel B. Gómez-Coca, Wenceslao Moreda, Ivan Best, María Isabel Cajo-Pinche, Juan Francisco Loja, Elena Ibañez, Alejandro Cifuentes, and Fernando Ramos-Escudero. 2022. "Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating Quality, Polyphenol Content and Antioxidant Activity" Foods 11, no. 10: 1518. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11101518