Long-Term Cryostorage of Mesenchymal Stem Cell-Containing Hybrid Hydrogel Scaffolds Based on Fibrin and Collagen
Abstract
:1. Introduction
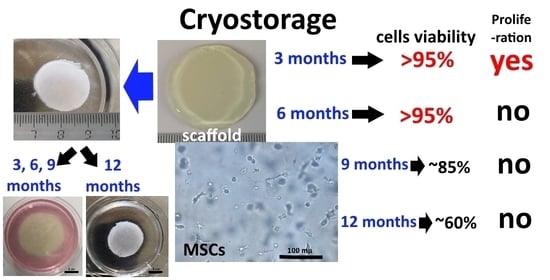
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Compliance with Legal Regulations and Ethical Norms
4.2. Hybrid Hydrogel Scaffolds
4.3. Cell Cultures
4.4. Cryostorage of Scaffolds
4.5. Microscopy
4.6. Quantitative Analysis of Cells in Scaffolds
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the Treatment of Chronic Wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towler, M.A.; Rush, E.W.; Richardson, M.K.; Williams, C.L. Randomized, Prospective, Blinded-Enrollment, Head-To-Head Venous Leg Ulcer Healing Trial Comparing Living, Bioengineered Skin Graft Substitute (Apligraf) with Living, Cryopreserved, Human Skin Allograft (TheraSkin). Clin. Podiatr. Med. Surg. 2018, 35, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue engineering for cutaneous wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R. The Efficacy and Safety of Dermagraft in. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y.J. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Martin-Piedra, M.A.; Alfonso-Rodriguez, C.A.; Zapater, A.; Durand-Herrera, D.; Chato-Astrain, J.; Campos, F.; Sanchez-Quevedo, M.C.; Alaminos, M.; Garzon, I. Effective use of mesenchymal stem cells in human skin substitutes generated by tissue engineering. Eur. Cell. Mater. 2019, 37, 233–249. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, B.; Yi, C.; Mo, X. Stem cell homing-based tissue engineering using bioactive materials. Front. Mater. Sci. 2017, 11, 93–105. [Google Scholar] [CrossRef]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.M.; Elabd, C.; Amri, E.Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Blanc, K.; Le Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- François, M.; Copland, I.B.; Yuan, S.; Romieu-Mourez, R.; Waller, E.K.; Galipeau, J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012, 14, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, K.; Sumstad, D.; Kadidlo, D.; McKenna, D.H.; Hubel, A. Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy 2015, 17, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oja, S.; Kaartinen, T.; Ahti, M.; Korhonen, M.; Laitinen, A.; Nystedt, J. The utilization of freezing steps in mesenchymal stromal cell (MSC) manufacturing: Potential impact on quality and cell functionality attributes. Front. Immunol. 2019, 10, 1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, M.; Sengupta, J.; Diaz-Dussan, D.; Adam, M.K.; Wu, M.; Acker, J.; Ben, R.; Ishihara, K.; Zeng, H.; Miura, Y.; et al. Synthesis of highly biocompatible and temperature-responsive physical gels for cryopreservation and 3D cell culture. ACS Appl. Bio Mater. 2018, 1, 356–366. [Google Scholar] [CrossRef]
- Diaz-Dussan, D.; Peng, Y.-Y.; Sengupta, J.; Zabludowski, R.; Adam, M.K.; Acker, J.P.; Ben, R.N.; Kumar, P.; Narain, R. Trehalose-Based Polyethers for Cryopreservation and Three-Dimensional Cell Scaffolds. Biomacromolecules 2020, 21, 1264–1273. [Google Scholar] [CrossRef]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel biomaterials for stem cell microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef] [Green Version]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Popa, E.G.; Rodrigues, M.T.; Coutinho, D.F.; Oliveira, M.B.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Cryopreservation of cell laden natural origin hydrogels for cartilage regeneration strategies. Soft Matter 2013, 9, 875–885. [Google Scholar] [CrossRef]
- Khetan, S.; Corey, O. Maintenance of stem cell viability and differentiation potential following cryopreservation within 3-dimensional hyaluronic acid hydrogels. Cryobiology 2019, 90, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Tapia, L.G.; Fohlerová, Z.; Žídek, J.; Alvarez-Perez, M.A.; Čelko, L.; Kaiser, J.; Montufar, E.B. Effects of cryopreservation on cell metabolic activity and function of biofabricated structures laden with osteoblasts. Materials 2020, 13, 1966. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, K.; Sugimoto, T.; Yamazaki, Y.; Takeda, A.; Uchinuma, E. Osteogenic potential, multipotency, and cytogenetic safety of human bone tissue-derived mesenchymal stromal cells (hBT-MSCs) after long-term cryopreservation. Orig. Contrib. Kitasato Med. J. 2014, 44, 95–103. [Google Scholar]
- Lechanteur, C.; Briquet, A.; Giet, O.; Delloye, O. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. BioMed Central 2016, 14, 145. [Google Scholar] [CrossRef] [Green Version]
- Gramlich, O.W.; Burand, A.J.; Brown, A.J.; Deutsch, R.J.; Kuehn, M.H.; Ankrum, J.A. Cryopreserved Mesenchymal Stromal Cells Maintain Potency in a Retinal Ischemia/ Reperfusion Injury Model: Toward an off-the- shelf Therapy. Sci. Rep. 2016, 6, 26463. [Google Scholar] [CrossRef]
- Chabot, D.; Lewin, A.; Loubaki, L.; Bazin, R. Functional impairment of MSC induced by transient warming events: Correlation with loss of adhesion and altered cell size. Cytotherapy 2018, 20, 990–1000. [Google Scholar] [CrossRef]
- Irdani, T.; Mazzanti, B.; Ballerini, L.; Saccardi, R.; Torre, R. A non-traditional approach to cryopreservation by ultra-rapid cooling for human mesenchymal stem cells. PLoS ONE 2019, 14, e0220055. [Google Scholar] [CrossRef] [Green Version]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Bhattacharya, S. Cryopretectants and Their Usage in Cryopreservation Process. Cryopreserv. Biotechnol. Biomed. Biol. Sci. 2018. [Google Scholar] [CrossRef]
- Meryman, H.T. Cryoprotective agents. Cryobiology 1971, 8, 173–183. [Google Scholar] [CrossRef]
- Davies, O.G.; Smith, A.J.; Cooper, P.R.; Shelton, R.M.; Scheven, B.A. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology 2014, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.I.; Wu, Q.; Wang, Y.; Li, L.I.; Bu, H.; Bao, J.I. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Münz, F.; Lopez Perez, R.; Trinh, T.; Sisombath, S.; Weber, K.J.; Wuchter, P.; Debus, J.; Saffrich, R.; Huber, P.E.; Nicolay, N.H. Human mesenchymal stem cells lose their functional properties after paclitaxel treatment. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rühle, A.; Huber, P.E.; Saffrich, R.; Lopez Perez, R.; Nicolay, N.H. The current understanding of mesenchymal stem cells as potential attenuators of chemotherapy-induced toxicity. Int. J. Cancer 2018, 143, 2628–2639. [Google Scholar] [CrossRef] [Green Version]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved mesenchymal stem cells regain functional potency following a 24-h acclimation period. J. Transl. Med. BioMed Central 2019, 17, 297. [Google Scholar] [CrossRef] [Green Version]
- Zander, J.; Bruegel, M.; Kleinhempel, A.; Becker, S.; Petros, S.; Kortz, L.; Dorow, J.; Kratzsch, J.; Baber, R.; Ceglarek, U.; et al. Effect of biobanking conditions on short-term stability of biomarkers in human serum and plasma. Clin. Chem. Lab. Med. 2014, 52, 629–639. [Google Scholar] [CrossRef]
- Gurruchaga, H.; Ciriza, J.; Saenz Del Burgo, L.; Rodriguez-Madoz, J.R.; Santos, E.; Prosper, F.; Hernández, R.M.; Orive, G.; Pedraz, J.L. Cryopreservation of microencapsulated murine mesenchymal stem cells genetically engineered to secrete erythropoietin. Int. J. Pharm. 2015, 485, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pravdyuk, A.I.; Petrenko, Y.A.; Fuller, B.J.; Petrenko, A.Y. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology 2013, 66, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Hydrogel cryopreservation system: An effective method for cell storage. Int. J. Mol. Sci. 2018, 19, 3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, E.; Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019, 4, 99–115. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. R. Soc. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Yang, Y.; Melzer, C.; Bucan, V.; Von Der Ohe, J.; Otte, A.; Hass, R. Conditioned umbilical cord tissue provides a natural three-dimensional storage compartment as in vitro stem cell niche for human mesenchymal stroma/stem cells. Stem Cell Res. Ther. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cagol, N.; Bonani, W.; Maniglio, D.; Migliaresi, C.; Motta, A. Effect of cryopreservation on cell-laden hydrogels: Comparison of different cryoprotectants. Tissue Eng. Part C Methods 2018, 24, 20–31. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Levin, G.Y.; Charykova, I.N.; Alejnik, D.Y.; Sosnina, L.N. Method for Creating a Bioresorbable Cellular Scaffold Based on Fibrin of Blood Plasma. Patent Application RU2653434C1, 18 May 2018. [Google Scholar]
- Egorikhina, M.N.; Charykova, I.N.; Alejnik, D.Y. Method of Quantitative Analysis of Cellular Components of Scaffold. Int. Cl. G01N 33/52 Bull. 35 Patent No. 2675376 RU, 19 December 2018. [Google Scholar]



| The Timing of the Cryostorage | Before Freezing (Total Number of Cell Nuclei per 1 mm3 Scaffold) | After Freezing (Total Number of Cell Nuclei per 1 mm3 Scaffold) | ||||
|---|---|---|---|---|---|---|
| 72 h | 144 h | Bb 24 h | Bb 96 h | DMSO 24 h | DMSO 96 h | |
| 3 months | 354.13 ± 18.16 | 616.53 ± 20.71 ○○ | 548.95 ± 19.21 ○ | 687.18 ± 19.48 ▪ ○ ● | 589.46 ± 26.98 ○○ | 636.07 ± 17.6 ◊ ○○ |
| 6 months | 338.16 ± 11.20 | 640.90 ± 15.40 ○○ | 513.26 ± 25.15 ○○ | 339.54 ± 18.48 ▪ ○ ● | 510.10 ± 21.89 ○ | 312.17 ± 18.29 ◊◊◊ ○○ ● |
| 9 months | 346.35 ± 12.03 | 787.91 ± 29.71 ○○ | 517.70 ± 10.76 ○○ | 327.17 ± 8.04 ▪ ○ | 428.76 ± 10.86 ○○ ∆ | 354.64 ± 16.11 ◊◊ ○ ● |
| 12 months | 369.46 ± 21.40 | 640.90 ± 14.85 ○○ | 587.83 ± 36.97 ○ | 592.82 ± 41.63 | 540.02 ± 21.51 ○ | 539.42 ± 21.78 ○ |
| The Timing of the Cryostorage | Before Freezing (Number of Nuclei of Dead Cells per 1 mm3 Scaffold) | After Freezing (Number of Nuclei of Dead Cells per 1 mm3 Scaffold) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 h | % | 144 h | % | Bb 24 h | % | Bb 96 h | % | DMSO 24 h | % | DMSO 96 h | % | |
| 3 months | 2.48 ± 0.64 | 0.70 | 2.64 ± 0.68 | 0.43 | 4.89 ± 0.94 | 0.89 | 7.99 ± 2.37 ○ | 1.16 | 4.58 ± 1.44 | 0.78 | 10.32 ± 2.09 ○ ● | 1.62 |
| 6 months | 1.24 ± 0.32 | 0.37 | 4.04 ± 1.04 ○ | 0.63 | 2.43 ± 1.70 | 0.47 | 4.38 ± 2.34 | 1.29 | 1.95 ± 0.86 | 0.38 | 3.89 ± 2.12 | 1.25 |
| 9 months | 2.56± 0.66 | 0.74 | 7.06 ± 1.82 | 0.90 | 43.59 ± 3.07 ○○ | 8.42 | 47.83 ± 7.44 ○○ | 14.62 | 50.30 ± 7.04 ○○ ● | 11.73 | 51.79 ± 9.94 ○○ ● | 14.60 |
| 12 months | 1.71 ± 0.44 | 0.46 | 3.82 ± 0.98 ○ | 0.59 | 205.35 ± 23.24 ○○ | 34.93 | 235.52 ± 27.75 ○○ | 39.73 | 241.36 ± 37.74 ○○ ●● | 44.69 | 264.2 ± 28.94 ○○ ●● | 48.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorikhina, M.N.; Rubtsova, Y.P.; Aleynik, D.Y. Long-Term Cryostorage of Mesenchymal Stem Cell-Containing Hybrid Hydrogel Scaffolds Based on Fibrin and Collagen. Gels 2020, 6, 44. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040044
Egorikhina MN, Rubtsova YP, Aleynik DY. Long-Term Cryostorage of Mesenchymal Stem Cell-Containing Hybrid Hydrogel Scaffolds Based on Fibrin and Collagen. Gels. 2020; 6(4):44. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040044
Chicago/Turabian StyleEgorikhina, Marfa N., Yulia P. Rubtsova, and Diana Ya. Aleynik. 2020. "Long-Term Cryostorage of Mesenchymal Stem Cell-Containing Hybrid Hydrogel Scaffolds Based on Fibrin and Collagen" Gels 6, no. 4: 44. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040044