Nanoporous Sodium Carboxymethyl Cellulose-g-poly (Sodium Acrylate)/FeCl3 Hydrogel Beads: Synthesis and Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
2.2. Thermal Gravimetric Analysis (TGA)
2.3. Morphological Analysis
2.4. Swelling Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CMC-g-PNaA
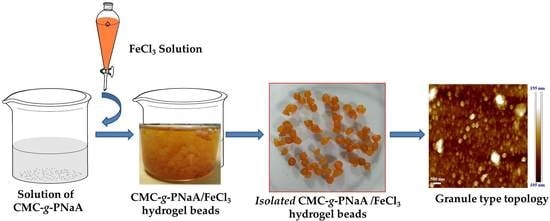
4.3. Preparation of CMC-g-PNaA/FeCl3 Hydrogel Beads
4.4. Characterization
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, B.; Negi, Y.S. To investigate the effect of ester-linkage on the properties of polyvinyl alcohol/carboxymethyl cellulose based hydrogel. Mater. Lett. 2019, 252, 308–312. [Google Scholar] [CrossRef]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Sauraj, R.; Bano, S.; Kumar, A.; Negi, Y.S. Development of novel cross-linked carboxymethyl cellulose/poly(potassium 1-hydroxy acrylate): Synthesis, characterization and properties. Polym. Bull. 2019. [CrossRef]
- Jayaramudu, T.; Ko, H.U.; Kim, H.C.; Kim, J.W.; Li, Y.; Kim, J. Transparent and semi-interpenetrating network P(vinyl alcohol)- P(Acrylic acid) hydrogels: pH responsive and electroactive application. Int. J. Smart Nano Mater. 2017, 8, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Rao, K.M.; Han, S.S. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydr. Polym. 2018, 193, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surfaces B Biointerfaces 2018, 170, 364–372. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, Y.; Liu, Q.; Zhong, Y.; Wang, Y.; Li, L.; Lincoln, S.F.; Guo, X. Preparation of a poly(acrylic acid) based hydrogel with fast adsorption rate and high adsorption capacity for the removal of cationic dyes. RSC Adv. 2019, 9, 21075–21085. [Google Scholar] [CrossRef] [Green Version]
- Elbedwehy, A.M.; Atta, A.M. Novel superadsorbent highly porous hydrogel based on arabic gum and acrylamide grafts for fast and efficient methylene blue removal. Polymers 2020, 12, 338. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Huang, S.; Li, X.; Zhang, L.; Tan, Y.; Wei, C.; Lv, J. Facile fabrication of novel polyhedral oligomeric silsesquioxane/carboxymethyl cellulose hybrid hydrogel based on supermolecular interactions. Mater. Lett. 2013, 90, 142–144. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.; Kalarikkal, N.; Elain, A.; Wui, T.; Thomas, S.; Grohens, Y. Wound healing analysis of pectin/carboxymethyl cellulose/micro fi brillated cellulose based composite scaffolds. Mater. Lett. 2014, 132, 34–37. [Google Scholar] [CrossRef]
- Salahinejad, E.; Hadianfard, M.J.; MacDonald, D.D.; Mozafari, M.; Vashaee, D.; Tayebi, L. Zirconium titanate thin film prepared by an aqueous particulate sol-gel spin coating process using carboxymethyl cellulose as dispersant. Mater. Lett. 2012, 88, 5–8. [Google Scholar] [CrossRef]
- Tiwari, N.; Nawale, L.; Sarkar, D.; Badiger, M. Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin. Gels 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühbeck, D.; Mayr, J.; Häring, M.; Hofmann, M.; Quignard, F.; Díaz Díaz, D. Evaluation of the nitroaldol reaction in the presence of metal ion-crosslinked alginates. New J. Chem. 2015, 39, 2306–2315. [Google Scholar] [CrossRef] [Green Version]
- Benhalima, T.; Ferfera-Harrar, H. Eco-friendly porous carboxymethyl cellulose/dextran sulfate composite beads as reusable and efficient adsorbents of cationic dye methylene blue. Int. J. Biol. Macromol. 2019, 132, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Hari, S.N.G.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Macromolecules Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Uva, M.; Mencuccini, L.; Atrei, A.; Innocenti, C.; Fantechi, E.; Sangregorio, C.; Maglio, M.; Fini, M.; Barbucci, R. On the Mechanism of Drug Release from Polysaccharide Hydrogels Cross-Linked with Magnetite Nanoparticles by Applying Alternating Magnetic Fields: The Case of DOXO Delivery. Gels 2015, 1, 24–43. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb ( II ) removal using sodium alginate—carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yuguchi, Y.; Stokke, B.T.; Sikorski, P.; Bassett, D.C. Local structure of Ca2+ alginate hydrogels gelled via competitive ligand exchange and measured by small angle X-ray scattering. Gels 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Zhang, Z.; Liu, Y.; Pang, M. Template-free synthesis and metalation of hierarchical covalent organic framework spheres for photothermal therapy. Chem. Commun. 2019, 55, 14315–14318. [Google Scholar] [CrossRef]
- Shi, Y.; Deng, X.; Bao, S.; Liu, B.; Liu, B.; Ma, P.; Cheng, Z.; Pang, M.; Lin, J. Self-Templated Stepwise Synthesis of Monodispersed Nanoscale Metalated Covalent Organic Polymers for In Vivo Bioimaging and Photothermal Therapy. Chem. Asian J. 2017, 12, 2183–2188. [Google Scholar] [CrossRef]
- Yang, S.; Fu, S.; Liu, H.; Zhou, Y.; Li, X. Hydrogel Beads Based on Carboxymethyl Cellulose for Removal Heavy Metal Ions. J. Appl. Polym. Sci. 2011, 119, 1204–1210. [Google Scholar] [CrossRef]
- Akalin, G.O.; Pulat, M. Preparation and Characterization of Nanoporous Sodium Carboxymethyl Cellulose Hydrogel Beads. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Rhim, J.W. Green and facile synthesis of carboxymethylcellulose/ZnO nanocomposite hydrogels crosslinked with Zn2+ ions. Int. J. Biol. Macromol. 2020, 162, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Klinpituksa, P.; Kosaiyakanon, P. Superabsorbent Polymer Based on Sodium Carboxymethyl Cellulose Grafted Polyacrylic Acid by Inverse Suspension Polymerization. Int. J. Polym. Sci. 2017, 1–7. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; He, C.; Kang, Y.; Zhou, J. Ionically crosslinked chitosan/poly (acrylic acid) hydrogels with high strength, toughness and antifreezing capability. Carbohydr. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis and characterization of superabsorbent hydrogels based on hydroxyethylcellulose and acrylic acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.; Wang, L.; Abdin, Z.; Yang, X.; Wang, J.; Zhou, W.; Zhang, H.; Chen, X. Synthesis and characterization of amylose grafted poly (acrylic acid) and its application in ammonia adsorption. Carbohydr. Polym. 2016, 153, 429–434. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Rahman, B.H.M.A.; Ali, B.A. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Van Tran, V.; Park, D.; Lee, Y.C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Castel, D.; Ricard, A.; Audebert, R.; De, L. Swelling of Anionic and Cationic Starch-Based Superabsorbents in Water and Saline Solution. J. Appl. Polym. Sci. 1990, 39, 11–29. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y. Synthesis of polypyrrole/sodium carboxymethyl cellulose nanospheres with enhanced supercapacitor performance. Mater. Lett. 2015, 139, 145–148. [Google Scholar] [CrossRef]
- Kumar, B.; Negi, Y.S. Synthesis and thermal properties of novel poly(potassium 1-hydroxy acrylate-co-potassium acrylate) based copolymer. Mater. Lett. 2018, 232, 196–201. [Google Scholar] [CrossRef]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Miao, W.; Cheng, W.; Wang, Z.; Wang, R.; Peng, J.; Zhu, Q. Influence of n-butyl acrylate and maleic anhydride copolymer on the structure and properties of phenolic resin. Mater. Today Commun. 2020, 23, 100879. [Google Scholar] [CrossRef]
- Kumar, B.; Negi, Y.S. Water absorption and viscosity behaviour of thermally stable novel graft copolymer of carboxymethyl cellulose and poly(sodium 1-hydroxy acrylate). Carbohydr. Polym. 2018, 181, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, G.; Liu, Y.; Long, D. Preparation of poly(acrylic acid) grafted multiwalled carbon nanotubes by a two-step irradiation technique. Macromolecules 2006, 39, 330–334. [Google Scholar] [CrossRef]
- Alosmanov, R.; Imanova, J.; Wolski, K.; Ziemmermann, R.; Fiejdasz, S.; Przewoźnik, J.; Goc, K.; Kapusta, C.; Zapotoczny, S.; Szuwarzyński, M. Fabrication of functional carbon/magnetic nanocomposites as a promising model of utilization of used crosslinked polymers. Materials 2018, 11, 2595. [Google Scholar] [CrossRef] [Green Version]
- Salama, A. Carboxymethyl cellulose-g-poly(acrylic acid)/calcium phosphate composite as a multifunctional hydrogel material. Mater. Lett. 2015, 157, 243–247. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Omer, A.M.; Soliman, E.A.; Hassan, E.A. Superabsorbent polyacrylamide grafted carboxymethyl cellulose pH sensitive hydrogel: I. Preparation and characterization. Desalin. Water Treat. 2013, 51, 3196–3206. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Harzandi, A.M.; Hosseinzadeh, H. Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur. Polym. J. 2004, 40, 1363–1370. [Google Scholar] [CrossRef]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Negi, Y.S. Agriculture: Super Absorbent Functional Polymers. Encycl. Polym. Appl. 2019, 3, 93–110. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, B.; Priyadarshi, R.; Sauraj; Deeba, F.; Kulshreshtha, A.; Gaikwad, K.K.; Kim, J.; Kumar, A.; Negi, Y.S. Nanoporous Sodium Carboxymethyl Cellulose-g-poly (Sodium Acrylate)/FeCl3 Hydrogel Beads: Synthesis and Characterization. Gels 2020, 6, 49. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040049
Kumar B, Priyadarshi R, Sauraj, Deeba F, Kulshreshtha A, Gaikwad KK, Kim J, Kumar A, Negi YS. Nanoporous Sodium Carboxymethyl Cellulose-g-poly (Sodium Acrylate)/FeCl3 Hydrogel Beads: Synthesis and Characterization. Gels. 2020; 6(4):49. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040049
Chicago/Turabian StyleKumar, Bijender, Ruchir Priyadarshi, Sauraj, Farha Deeba, Anurag Kulshreshtha, Kirtiraj K. Gaikwad, Jaehwan Kim, Anuj Kumar, and Yuvraj Singh Negi. 2020. "Nanoporous Sodium Carboxymethyl Cellulose-g-poly (Sodium Acrylate)/FeCl3 Hydrogel Beads: Synthesis and Characterization" Gels 6, no. 4: 49. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040049