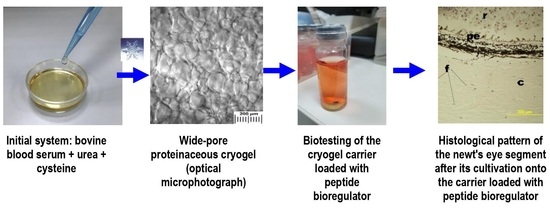
Cryostructuring of Polymeric Systems. 57. Spongy Wide-Porous Cryogels Based on the Proteins of Blood Serum: Preparation, Properties and Application as the Carriers of Peptide Bioregulators
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Proteinaceous Cryogels and Their Characterization
2.2. Testing of Proteinaceous Cryogels as the Carriers of Peptide Bioregulators
3. Experimental
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Wide-Porous Cryogels Based on the Blood Serum Proteins
3.2.2. Characterization of the Synthesized Cryogels
3.2.3. Microstructure of Proteinaceous Cryogels
3.2.4. Component Composition of the Cryogels Based on Blood Serum Proteins
3.2.5. Preparation of the Bioregulator-Loaded Cryogels and Reference Samples
3.2.6. Preparation of Biological Samples
3.2.7. Cultivation Experiments and Analysis of the Resulting Biological Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Mikos, A.G. Biomaterials: The Intersection of Biology and Materials Science; Pearson/Prentice Hall: New York, NJ, USA, 2008; p. 478. ISBN 978-0132-35044-0. [Google Scholar]
- Narayan, R. Biomedical Materials; Springer: Boston, MA, USA, 2009; p. 546. ISBN 978-0-387-84871-6. [Google Scholar]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Vlierberghe, S.V.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; La Cruz, D.S.-D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [Green Version]
- Shtilman, M.I. Polymeric Biomaterials. In Part I. Polymer Implants; VSP BV: Utrecht, The Netherlands, 2003; p. 276. ISBN 90-6764-389-0. [Google Scholar]
- Godbey, W.T. An Introduction to Biotechnology; Academic Press: Amsterdam, The Netherlands, 2015; p. 436. ISBN 978-1-90756-828-2. [Google Scholar]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Kirsebom, H.; Mattiasson, B. Cryostructuration as a tool for preparing highly porous polymer materials. Polym. Chem. 2011, 2, 1059–1062. [Google Scholar] [CrossRef]
- Gun’Ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Okay, O. Basic Principles of Cryotropic Gelation. Adv. Polym. Sci. 2014, 263, 49–101. [Google Scholar] [CrossRef]
- Liu, C.; Tong, G.; Chen, C.; Tan, Z.; Quan, C.; Zhang, C. Polymeric cryogel: Preparation, properties and biomedical applications. Progr. Chem. 2014, 26, 1190–1201. (In Chinese) [Google Scholar] [CrossRef]
- Okay, O. Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Springer: Cham, Switzerland, 2014; p. 330. ISBN 978-3-319-05845-0. [Google Scholar]
- Kumar, A. Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2016; p. 480. ISBN 978-1-4822-281-6. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Lagoyanni, T. Immobilization of photosynthetically active cyanobacteria in glutaraldehyde-crosslinked albumin matrix. Appl. Microbiol. Biotechnol. 1986, 23, 417–423. [Google Scholar] [CrossRef]
- Carpentier, R.; Lemieux, S.; Mimeault, M. Photocurrent genration by thylakoid membranes immobilized in an albumin-glutaraldehyde cross-linked matrix. Biotechnol. Lett. 1988, 10, 133–136. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Konstantinova, N.R.; Solov’eva, N.I. Method for the Preparation of Porous Protein Gel. Russian Patent 2,058,083, 20 April 1996. [Google Scholar]
- Konstantinova, N.R.; Lozinsky, V.I. Cryotropic gelation of ovalbumin solutions. Food Hydrocoll. 1997, 11, 113–123. [Google Scholar] [CrossRef]
- Shimoyamada, M.; Tômatsu, K.; Watanabe, K. Effect of Precooling Step on Formation of Soymilk Freeze-Gel. Food Sci. Technol. Res. 1999, 5, 284–288. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, T. Poly(aspartic acid) Super-Absorbent Resin Produced by Chemical Crosslinking and Physical Freeze/Thawing. Macromol. Chem. Phys. 2006, 207, 1297–1305. [Google Scholar] [CrossRef]
- Hedström, M.; Plieva, F.; Galaev, I.Y.; Mattiasson, B. Monolithic macroporous albumin/chitosan cryogel structure: A new matrix for enzyme immobilization. Anal. Bioanal. Chem. 2007, 390, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Elowsson, L.; Kirsebom, H.; Carmignac, V.; Durbeej, M.; Mattiasson, B. Porous protein-based scaffolds prepared through freezing as potential scaffolds for tissue engineering. J. Mater. Sci. Mater. Electron. 2012, 23, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Lozinsky, V.I. Cryostructuring of polymer systems. Proteinaceous wide-pore cryogels generated by the action of denaturant/reductant mixtures on bovine serum albumin in moderately frozen aqueous media. Soft Matter 2015, 11, 4921–4931. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Lozinsky, V.I. Study of cryostructuring of polymeric systems. 42. Physical-chemical properties and microstructure of wide-porous covalently cross-linked albumin cryogels. Colloid J. 2016, 78, 492–504. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Shabatina, T.I.; Lozinsky, V.I. Cryostructuring of polymer systems. 44. Freeze-dried and then chemically cross-linked wide porous cryostructurates based on serum albumin. e-Polymers 2017, 17, 263–274. [Google Scholar] [CrossRef]
- Tykhvynska, O.A.; Rogulska, O.Y.; Volkova, N.O.; Grischuk, V.P.; Revenko, O.B.; Mazur, S.P.; Lozinsky, V.I.; Petrenko, Y.O.; Petrenko, O.Y. Blood Plasma-Based Macroporous Scaffolds as Biocompatible Coatings to Restore Full-Thickness Excision Wounds. Probl. Cryobiol. Cryomedicine 2018, 28, 44–48. [Google Scholar] [CrossRef]
- Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. Macroporous elastic cryogels based on platelet lysate and oxidized dextran as tissue engineering scaffold: In vitro and in vivo evaluations. Mater. Sci. Eng. C 2020, 110, 110703. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Shchekoltsova, A.O.; Sinitskaya, E.S.; Vernaya, O.I.; Nuzhdina, A.V.; Bakeeva, I.V.; Ezernitskaya, M.G.; Semenov, A.M.; Shabatina, T.I.; Melnikov, M.Y. Influence of succinylation of a wide-pore albumin cryogels on their properties, structure, biodegradability, and release dynamics of dioxidine loaded in such spongy carriers. Int. J. Biol. Macromol. 2020, 160, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Rodionov, I.A.; Tsiskarashvili, A.V.; Es’kin, N.A. Antibacterial Protein Sponge for Chemotherapy of Infected Wounds and Method of Its Preparation. Russian Patent 2,637,634, 5 December 2017. [Google Scholar]
- Anisimov, V.N.; Khavinson, V.K. Peptide bioregulation of aging: Results and prospects. Biogerontology 2009, 11, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, B.I.; Davydov, S.O.; Popravka, E.S.; Linkova, N.S.; Kozina, L.S.; Khavinson, V.K. Epigenetic Mechanisms of Peptide-Driven Regulation and Neuroprotective Protein FKBP1b. Mol. Biol. 2019, 53, 299–307. [Google Scholar] [CrossRef]
- Varfolomeev, S.D.; Burlakova, E.B.; Popov, A.A.; Zaikov, G.E. Biochemical Physics: Frontal Research; Nova Science Publishers, Inc.: New York, NY, USA, 2007; pp. 21–78. ISBN 978-1-60021-425-7. [Google Scholar]
- Yamskov, I.A.; Blagodatskikh, I.; Krasnov, M.S.; Borisenko, A.V.; Margasyuk, D.V.; Vecherkin, V.V.; Skripnikova, V.S.; Nazarova, P.A.; Bitko, S.A.; Berezin, B.B.; et al. Physicochemical properties of a new group of regulatory proteins isolated from various mammal tissues. Russ. Chem. Bull. 2009, 58, 640–645. [Google Scholar] [CrossRef]
- Krasnov, M.S.; Shaikhaliev, A.I.; Korshakov, E.V.; Efimenko, M.V.; Soloshenkov, P.P.; Davidova, T.R.; Zvukova, N.D.; Sinitskaya, E.S.; Yamskova, V.P.; Yamskov, I.A.; et al. Induction of Osteogenesis in Rat Bone Tissue Using Cryogenically Structured Porous 3D Materials Containing a Bioregulator. Bull. Exp. Biol. Med. 2019, 168, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Yamskova, V.P.; Reznikova, M.M. Low-molecular-weight peptide from the blood serum of homeothermic animals: Effect on cell adhesion and proliferation. Zhurnal Obshchei Biologii 1991, 52, 181–191. (In Russian) [Google Scholar]
- Skripnikova, V.S.; Krasnov, M.S.; Beresin, B.B.; Babushkina, T.A.; Borisenko, A.V.; Izmailov, B.A.; Yamskova, V.P.; Yamskov, I.A. Low-molecular-weight sclera protein biologically active at ultralow doses. Dokl. Biochem. Biophys. 2007, 417, 346–347. [Google Scholar] [CrossRef]
- Krasnov, M.S.; Grigoryan, E.N.; Yamskova, V.P. An Organotypic Culture of the Newt Retina together with Other Tissues of the Posterior Eye Segment as a Model for Studying the Effects of Cell Adhesion Glycoproteins. Biol. Bull. 2003, 30, 17–29. [Google Scholar] [CrossRef]
- Savina, I.N.; Tomlins, P.E.; Mikhalovsky, S.V.; Galaev, I.Y. Characterization of macroporous gels. In Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I.Y., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 211–236. ISBN 978-1-4200-8461-9. [Google Scholar]
- Okay, O.; Lozinsky, V.I. Synthesis and structure–property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering. Acta Biomater. 2016, 62, 29–41. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Ferrer, M.L.; Monte, F.D. Ice-Templated Materials: Sophisticated Structures Exhibiting Enhanced Functionalities Obtained after Unidirectional Freezing and Ice-Segregation-Induced Self-Assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Reichelt, S. Introduction to Macroporous Cryogels. Bioinform. MicroRNA Res. 2015, 1286, 173–181. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Ghalkhani, M.; Maleki, H. Directional Freeze-Casting: A Bioinspired Method to Assemble Multifunctional Aligned Porous Structures for Advanced Applications. Adv. Eng. Mater. 2020, 22, 2000033. [Google Scholar] [CrossRef]
- Azzazy, E.; Christenson, R.H. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: Cambridge, MA, USA, 1996; p. 432. ISBN 0-12-552110-3. [Google Scholar]
- Putman, F.W. The Plasma Proteins, 2nd ed.; Academic Press Inc.: Amsterdam, The Netherlands, 1975; Volume 1, p. 497. ISBN 978-0323-13808-6. [Google Scholar]
- Görg, A.; Postel, W.; Weser, J.; Schiwara, H.W.; Boesken, W.H. Horisontal SDS electrophoresis in ultrathin pore-gradient gels for the analysis of proteins. Sci. Tools 1985, 12, 5–9. [Google Scholar]
- Keren, D.F. Electrophoresis in Clinical Diagnosis; Edward Arnold Ltd.: London, UK, 2003; p. 256. ISBN 978-0340-81213-6. [Google Scholar]
- Agarwal, P.; Parkash, A.; Tejwani, N.; Mehta, A. Bisalbuminemia: A Rare Finding on Serum Electrophoresis. Indian J. Hematol. Blood Transfus. 2018, 34, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Sidorskii, E.V.; Il’Ina, A.P.; Krasnova, M.S.; Yamskova, V.P.; Buryak, A.K.; Yamskov, I.A. Physicochemical Properties and Biological Activity of the Peptide–Protein Complex from Bovine Scleral Tissue. Appl. Biochem. Microbiol. 2018, 54, 83–88. [Google Scholar] [CrossRef]
- Li, H.-H.; Huo, L.-J.; Gao, Z.-Y.; Zhao, F.; Zeng, J.-W. Regulation of scleral fibroblast differentiation by bone morphogenetic protein-2. Int. J. Ophthalmol. 2014, 7, 152–156. [Google Scholar]
- Hsiao, Y.-T.; Chang, W.-A.; Kuo, M.-T.; Lo, J.; Lin, H.-C.; Yen, M.-C.; Jian, S.-F.; Chen, Y.-J.; Kuo, P.-L. Systematic Analysis of Transcriptomic Profile of the Effects of Low Dose Atropine Treatment on Scleral Fibroblasts using Next-Generation Sequencing and Bioinformatics. Int. J. Med. Sci. 2019, 16, 1652–1667. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 672. ISBN 978-0-7020-6864-5. [Google Scholar]
- Richardson, A.M. Nonparametric Statistics: A Step-by-Step Approach. Int. Stat. Rev. 2015, 83, 163–164. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorskii, E.V.; Krasnov, M.S.; Yamskova, V.P.; Lozinsky, V.I. Cryostructuring of Polymeric Systems. 57. Spongy Wide-Porous Cryogels Based on the Proteins of Blood Serum: Preparation, Properties and Application as the Carriers of Peptide Bioregulators. Gels 2020, 6, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040050
Sidorskii EV, Krasnov MS, Yamskova VP, Lozinsky VI. Cryostructuring of Polymeric Systems. 57. Spongy Wide-Porous Cryogels Based on the Proteins of Blood Serum: Preparation, Properties and Application as the Carriers of Peptide Bioregulators. Gels. 2020; 6(4):50. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040050
Chicago/Turabian StyleSidorskii, Egor V., Mikhail S. Krasnov, Viktoria P. Yamskova, and Vladimir I. Lozinsky. 2020. "Cryostructuring of Polymeric Systems. 57. Spongy Wide-Porous Cryogels Based on the Proteins of Blood Serum: Preparation, Properties and Application as the Carriers of Peptide Bioregulators" Gels 6, no. 4: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/gels6040050