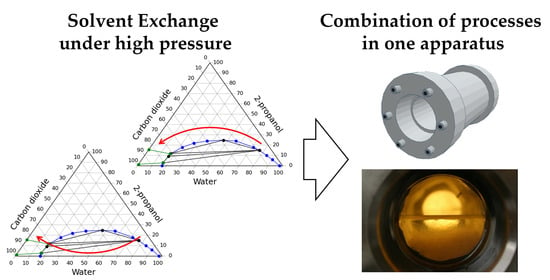
Investigation of Aerogel Production Processes: Solvent Exchange under High Pressure Combined with Supercritical Drying in One Apparatus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Theoretical Investigation of ‘Carbon Dioxide–Water–2-Propanol’ Ternary Sistem
2.2. Experimental Investigation of the Solvent Exchange Process under High Pressure
2.2.1. Solvent Exchange through Multiphase Region of Phase Diagram
- experiments 1 and 2—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–70–90–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 10% to achieve a pressure of 80 bar;
- experiments 3 and 4—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–70–80–90–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 10% to achieve a pressure of 80 bar;
- experiments 5 and 6—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–70–80–90–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 10% to achieve a pressure of 80 bar;
- experiments 7—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–70–80–90–100–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 50% to achieve a pressure of 80 bar;
- experiments 8—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–70–90–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 10% to achieve a pressure of 80 bar.
2.2.2. Solvent Exchange through Homogeneous Region of Phase Diagram
- experiment 9—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–70–80–90–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 50%; at each step starting from a step of 50%, the pressure in the apparatus increased as follows: 10–20–30–40–50 bar;
- experiment 10—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–70–80–90–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 50%; at each step starting from a step of 50%, the pressure in the apparatus increased as follows: 10–20–30–80–80 bar;
- experiment 11—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–70–80–90–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 50%; at each step starting from a step of 50%, the pressure in the apparatus increased as follows: 10–20–50–60–90 bar.
- experiment 12—the composition was changed to follow mass concentration of 2-propanol sequence 10–30–50–60–80–90–95–100–100 wt.%; carbon dioxide feed was started at a step when mass concentration of alcohol was 50%; at each step starting from a step of 50%, the pressure in the apparatus increased as follows: 10–20–30–80–80–80 bar;
2.3. Intensification of the Solvent Exchange Process under High Pressure
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Sodium Alginate Gels
4.3. Method of the Investigation of Solvent Exchange under High Pressure
- Supply of a given amount of 2-propanol to reach the desired concentration according to stepwise solvent exchange procedure;
- Supply of the required amount of carbon dioxide to achieve or maintain the specified pressure;
- Draining part of “carbon dioxide–water–2-propanol” medium from the apparatus.
4.4. Analytical Methods
4.5. Calculation of the Solvent Exchange Operating Line
- algorithm for processing experimental data, which allows to build experimental operating lines of the process on phase diagrams;
- algorithm for calculating the theoretical operating lines of the solvent exchange process and plotting them on phase diagrams.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction. Available online: https://0-www-elsevier-com.brum.beds.ac.uk/books/supercritical-fluid-extraction/mchugh/978-0-08-051817-6 (accessed on 25 November 2020).
- Türk, M.; Bolten, D. Polymorphic Properties of Micronized Mefenamic Acid, Nabumetone, Paracetamol and Tolbutamide Produced by Rapid Expansion of Supercritical Solutions (RESS). J. Supercrit. Fluids 2016, 116, 239–250. [Google Scholar] [CrossRef]
- Bartle, K.D.; Davies, I.L.; Raynor, M.W. Supercritical fluid chromatography. In Size Exclusion Chromatography; Hunt, B.J., Holding, S.R., Eds.; Springer: Boston, MA, USA, 1989; pp. 217–247. [Google Scholar]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical Impregnation of Lavandin (Lavandula Hybrida) Essential Oil in Modified Starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical Drying of Aerogels Using CO2: Effect of Extraction Time on the End Material Textural Properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Griffin, J.S.; Mills, D.H.; Cleary, M.; Nelson, R.; Manno, V.P.; Hodes, M. Continuous Extraction Rate Measurements during Supercritical CO2 Drying of Silica Alcogel. J. Supercrit. Fluids 2014, 94, 38–47. [Google Scholar] [CrossRef]
- Parale, V.G.; Lee, K.-Y.; Park, H.-H. Flexible and Transparent Silica Aerogels: An Overview. J. Korean Ceram. Soc. 2017, 54, 184–199. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Zhong, X.; Ding, Y.; Zhang, S.; Shi, F.; Hu, J. Alginate-Based Aerogels with Double Catalytic Activity Sites and High Mechanical Strength. Carbohydr. Polym. 2020, 245, 116490. [Google Scholar] [CrossRef] [PubMed]
- Rubina, M.S.; Elmanovich, I.V.; Shulenina, A.V.; Peters, G.S.; Svetogorov, R.D.; Egorov, A.A.; Naumkin, A.V.; Vasil’kov, A.Y. Chitosan Aerogel Containing Silver Nanoparticles: From Metal-Chitosan Powder to Porous Material. Polym. Test. 2020, 86, 106481. [Google Scholar] [CrossRef]
- Parale, V.G.; Kim, T.; Lee, K.-Y.; Phadtare, V.D.; Dhavale, R.P.; Jung, H.-N.-R.; Park, H.-H. Hydrophobic TiO2–SiO2 Composite Aerogels Synthesized via in Situ Epoxy-Ring Opening Polymerization and Sol-Gel Process for Enhanced Degradation Activity. Ceram. Int. 2020, 46, 4939–4946. [Google Scholar] [CrossRef]
- Lebedev, A.E.; Menshutina, N.V.; Khudeev, I.I.; Kamyshinsky, R.A. Investigation of Alumina Aerogel Structural Characteristics at Different «precursor-Water-Ethanol» Ratio. J. Non-Cryst. Solids 2020, 120475. [Google Scholar] [CrossRef]
- Amonette, J.E.; Matyáš, J. Functionalized Silica Aerogels for Gas-Phase Purification, Sensing, and Catalysis: A Review. Microporous Mesoporous Mater. 2017, 250, 100–119. [Google Scholar] [CrossRef]
- Patil, S.P.; Parale, V.G.; Park, H.-H.; Markert, B. Molecular Dynamics and Experimental Studies of Nanoindentation on Nanoporous Silica Aerogels. Mater. Sci. Eng. A 2019, 742, 344–352. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Ghica, M.E.; Durães, L. An Overview on Alumina-Silica-Based Aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef] [PubMed]
- Najberg, M.; Haji Mansor, M.; Taillé, T.; Bouré, C.; Molina-Peña, R.; Boury, F.; Cenis, J.L.; Garcion, E.; Alvarez-Lorenzo, C. Aerogel Sponges of Silk Fibroin, Hyaluronic Acid and Heparin for Soft Tissue Engineering: Composition-Properties Relationship. Carbohydr. Polym. 2020, 237, 116107. [Google Scholar] [CrossRef] [PubMed]
- Aghabararpour, M.; Mohsenpour, M.; Motahari, S.; Abolghasemi, A. Mechanical Properties of Isocyanate Crosslinked Resorcinol Formaldehyde Aerogels. J. Non Cryst. Solids 2018, 481, 548–555. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of Macroporous Alginate-Based Aerogels for Biomedical Applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of Novel Whey Protein-Based Aerogels as Drug Carriers for Life Science Applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Raman, S.P.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H.; et al. Alginate Aerogels Carrying Calcium, Zinc and Silver Cations for Wound Care: Fabrication and Metal Detection. J. Supercrit. Fluids 2019, 153, 104545. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Thermal Conductivity/Structure Correlations in Thermal Super-Insulating Pectin Aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Angkawijaya, A.E.; Ju, Y.-H.; Tran-Nguyen, P.L. A One-Pot Synthesis of Biodiesel from Leather Tanning Waste Using Supercritical Ethanol: Process Optimization. Biomass Bioenergy 2020, 142, 105761. [Google Scholar] [CrossRef]
- Bahadori, A.; Vuthaluru, H.B.; Mokhatab, S. New Correlations Predict Aqueous Solubility and Density of Carbon Dioxide. Int. J. Greenh. Gas Control 2009, 3, 474–480. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2000; ISBN 978-0-07-011682-5. [Google Scholar]
- Haghbakhsh, R.; Hayer, H.; Saidi, M.; Keshtkari, S.; Esmaeilzadeh, F. Density Estimation of Pure Carbon Dioxide at Supercritical Region and Estimation Solubility of Solid Compounds in Supercritical Carbon Dioxide: Correlation Approach Based on Sensitivity Analysis. Fluid Phase Equilibria 2013, 342, 31–41. [Google Scholar] [CrossRef]
- Ouyang, L.-B. New Correlations for Predicting the Density and Viscosity of Supercritical Carbon Dioxide Under Conditions Expected in Carbon Capture and Sequestration Operations. Open Pet. Eng. J. 2011, 4. [Google Scholar] [CrossRef] [Green Version]
- Bahadori, A.; Vuthaluru, H.B. Predictive Tool for an Accurate Estimation of Carbon Dioxide Transport Properties. Int. J. Greenh. Gas Control 2010, 4, 532–536. [Google Scholar] [CrossRef]
- Heidaryan, E.; Hatami, T.; Rahimi, M.; Moghadasi, J. Viscosity of Pure Carbon Dioxide at Supercritical Region: Measurement and Correlation Approach. J. Supercrit. Fluids 2011, 56, 144–151. [Google Scholar] [CrossRef]
- Kestin, J.; Korfali, Ö.; Sengers, J.V. Density Expansion of the Viscosity of Carbon Dioxide near the Critical Temperature. Phys. Stat. Mech. Appl. 1980, 100, 335–348. [Google Scholar] [CrossRef]
- Zabaloy, M.S.; Vasquez, V.R.; Macedo, E.A. Viscosity of Pure Supercritical Fluids. J. Supercrit. Fluids 2005, 36, 106–117. [Google Scholar] [CrossRef]
- Alder, B.J.; Alley, W.E.; Dymond, J.H. Studies in Molecular Dynamics. XIV. Mass and Size Dependence of the Binary Diffusion Coefficient. J. Chem. Phys. 1974, 61, 1415–1420. [Google Scholar] [CrossRef]
- Catchpole, O.J.; King, M.B. Measurement and Correlation of Binary Diffusion Coefficients in Near Critical Fluids. Ind. Eng. Chem. Res. 1994, 33, 1828–1837. [Google Scholar] [CrossRef]
- Funazukuri, T.; Kong, C.Y.; Kagei, S. Effects of Molecular Weight and Degree of Unsaturation on Binary Diffusion Coefficients for Lipids in Supercritical Carbon Dioxide. Fluid Phase Equilibria 2004, 219, 67–73. [Google Scholar] [CrossRef]
- He, C.-H. Prediction of Binary Diffusion Coefficients of Solutes in Supercritical Solvents. AlChE J. 1997, 43, 2944–2947. [Google Scholar] [CrossRef]
- Kooijman, H.A. A Modification of the Stokes−Einstein Equation for Diffusivities in Dilute Binary Mixtures. Ind. Eng. Chem. Res. 2002, 41, 3326–3328. [Google Scholar] [CrossRef]
- Lai, C.-C.; Tan, C.-S. Measurement of Molecular Diffusion Coefficients in Supercritical Carbon Dioxide Using a Coated Capillary Column. Ind. Eng. Chem. Res. 1995, 34, 674–680. [Google Scholar] [CrossRef]
- Sassiat, P.R.; Mourier, P.; Caude, M.H.; Rosset, R.H. Measurement of Diffusion Coefficients in Supercritical Carbon Dioxide and Correlation with the Equation of Wilke and Chang. Anal. Chem. 1987, 59, 1164–1170. [Google Scholar] [CrossRef]
- Heidaryan, E.; Jarrahian, A. Modified Redlich-Kwong Equation of State for Supercritical Carbon Dioxide. J. Supercrit. Fluids 2013, 81, 92–98. [Google Scholar] [CrossRef]
- Frey, K.; Modell, M.; Tester, J. Density-and-Temperature-Dependent Volume Translation for the SRK EOS: 1. Pure Fluids. Fluid Phase Equilibria 2009, 279, 56–63. [Google Scholar] [CrossRef]
- Abudour, A.M.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. Volume-Translated Peng–Robinson Equation of State for Saturated and Single-Phase Liquid Densities. Fluid Phase Equilibria 2012, 335, 74–87. [Google Scholar] [CrossRef]
- Patel, N.C.; Teja, A.S. A New Cubic Equation of State for Fluids and Fluid Mixtures. Chem. Eng. Sci. 1982, 37, 463–473. [Google Scholar] [CrossRef]
- Du, G.; Hu, J. An Equation of State for Accurate Thermodynamic Modeling of Water and Carbon Dioxide from Triple Points to 647 K and 100–200 MPa. Int. J. Greenh. Gas Control 2016, 49, 94–107. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Weare, J. An Equation of State for the CH4-CO2-H2O System: I. Pure Systems from 0 to 1000 °C and 0 to 8000 Bar. Geochim. Cosmochim. Acta 1992. [Google Scholar] [CrossRef]
- Wendland, M.; Hasse, H.; Maurer, G. Multiphase High-Pressure Equilibria of Carbon Dioxide-Water-Isopropanol. J. Supercrit. Fluids 1993, 6, 211–222. [Google Scholar] [CrossRef]
- Menshutina, N.V.; Lebedev, A.E.; Lovskaya, D.D. The resource and energy saving process of aerogel particles obtainment based on the combination of the stages of solvent replacement and supercritical drying in one apparatus. Him. Promyshlennost Segodnya Chem. Ind. Today 2019, 2, 50–55. [Google Scholar]
- Lovskaya, D.; Menshutina, N. Alginate-Based Aerogel Particles as Drug Delivery Systems: Investigation of the Supercritical Adsorption and In Vitro Evaluations. Materials 2020, 13, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, P.; Viganó, J.; Furtado, G.F.; Cunha, R.L.; Hubinger, M.D.; Rezende, C.A.; Martínez, J. Production of Resveratrol Loaded Alginate Aerogel: Characterization, Mathematical Modeling, and Study of Impregnation. J. Supercrit. Fluids 2020, 163, 104882. [Google Scholar] [CrossRef]
- Şahin, İ.; Uzunlar, E.; Erkey, C. Investigation of the Effect of Gel Properties on Supercritical Drying Kinetics of Ionotropic Alginate Gel Particles. J. Supercrit. Fluids 2019, 152, 104571. [Google Scholar] [CrossRef]
- Menshutina, N.V.; Lovskaya, D.D.; Lebedev, A.E.; Lebedev, E.A. Production of Sodium Alginate-Based Aerogel Particles Using Supercritical Drying in Units with Different Volumes. Russ. J. Phys. Chem. B 2017, 11, 1296–1305. [Google Scholar] [CrossRef]
- Lebedev, A.E.; Katalevich, A.M.; Menshutina, N.V. Modeling and Scale-up of Supercritical Fluid Processes. Part I: Supercritical Drying. J. Supercrit. Fluids 2017, 106. [Google Scholar] [CrossRef]
- Tsygankov, P.Y.; Khudeev, I.I.; Lebedev, A.E.; Lebedev, E.A.; Menshutina, N.V. Lab Scale High-Pressure Equipment for Supercritical Drying. Chem. Eng. Trans. 2018, 70, 877–882. [Google Scholar] [CrossRef]









| No. | Name | Equations | Description | Reference |
|---|---|---|---|---|
| 1 | Modified Redlich–Kwong for carbon dioxide | At temperatures from 305 to 1100 K and pressures from 7.645 to 800 MPa, the deviation of the calculated data is relatively small (13%) | [37] | |
| 2 | Soave–Redlich–Kwong for non-polar substances | At pressures from 3 to 12 MPa, the average deviation of the calculated data from the experimental is 4.4% | [38] | |
| 3 | Peng–Robinson equation | In the near-critical temperature range, the error is 4.4% | [39] | |
| 4 | Patel–Teja equation | —smallest positive root of an equation: | In the near-critical region of temperatures and pressures, the average deviation of the calculated data from the experimental is 4.48% | [40] |
| 5 | Equation of state for near-critical and critical regions | At temperatures from 304 to 647 K and pressures up to 80 MPa, the deviation of the calculated data from the experimental was less than 1% | [41] | |
| 6 | Modified Lee–Kesler equation | At temperatures from 273 to 1273 K and pressures up to 350 MPa, the deviation of the calculated data from the experimental is insignificant (up to 0.5%) | [42] |
| No. | Name | Equations | Description |
|---|---|---|---|
| 1 | Van der Waals mixing rule | —empirical binary interaction parameter | Used for a wide range of substances |
| 2 | Mixing rule | This type of equations is used to calculate when mixing non-polar substances with polar or associated components. | |
| 3 | Huron–Vidal mixing rule | —excess free Gibbs energy The parameter C (V) depends on the chosen equation of state, for example, for the Peng-Robinson equation of state it is –0.6232 | Mixing rules based on the calculation of the thermodynamic potentials of the system |
| No. | 2-Propanol Consumption, g | CO2 Consumption, g | Gel Shrinkage, % |
|---|---|---|---|
| 1 | 261 | 764 | 55.4 |
| 2 | 237 | 581 | 57.3 |
| 3 | 387 | 659 | 53.8 |
| 4 | 250 | 647 | 74.9 |
| 5 | 398 | 767 | 56.5 |
| 6 | 336 | 947 | 58.2 |
| 7 | 288 | 794 | 60.2 |
| 8 | 287 | 964 | 65.0 |
| No. | 2-Propanol Consumption, g | CO2 Consumption, g | Gel Shrinkage, % |
|---|---|---|---|
| 9 | 275 | 62 | - |
| 10 | 268 | 241 | - |
| 11 | 642 | 341 | - |
| 12 | 414 | 441 | 5.9 |
| No. | ρbulk | Surface Area m2/g | Average Pore Diameter, nm | Ref. |
|---|---|---|---|---|
| 1 | 0.053 | 494 | 24 | This study |
| 2 | - | 512 | 27 | [45] |
| 3 | - | 499–598 | - | [19] |
| 4 | - | 381–425 | 7.2–8.1 | [46] |
| 5 | 0.05–0.08 | 236–449 | 12–19 | [47] |
| No. | 2-Propanol Consumption, g | CO2 Consumption, g |
|---|---|---|
| 1 | 765 | 800 |
| 2 | 530 | 813 |
| 3 | 446 | 856 |
| 4 | 424 | 783 |
| 5 | 368 | 704 |
| 6 | 217 | 243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, A.; Suslova, E.; Troyankin, A.; Lovskaya, D. Investigation of Aerogel Production Processes: Solvent Exchange under High Pressure Combined with Supercritical Drying in One Apparatus. Gels 2021, 7, 4. https://0-doi-org.brum.beds.ac.uk/10.3390/gels7010004
Lebedev A, Suslova E, Troyankin A, Lovskaya D. Investigation of Aerogel Production Processes: Solvent Exchange under High Pressure Combined with Supercritical Drying in One Apparatus. Gels. 2021; 7(1):4. https://0-doi-org.brum.beds.ac.uk/10.3390/gels7010004
Chicago/Turabian StyleLebedev, Artem, Ekaterina Suslova, Aleksander Troyankin, and Daria Lovskaya. 2021. "Investigation of Aerogel Production Processes: Solvent Exchange under High Pressure Combined with Supercritical Drying in One Apparatus" Gels 7, no. 1: 4. https://0-doi-org.brum.beds.ac.uk/10.3390/gels7010004