Genetic Regulation of Avian Testis Development
Abstract
:1. Introduction
2. Avian Sex Determination
3. Structure of the Adult Avian Testis
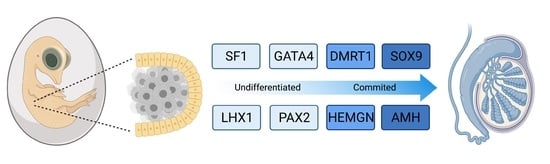
4. Embryonic Development of the Testis in the Chicken Model
5. Molecular Regulation of Avian Testis Formation and the Role of DMRT1
6. The Sertoli Cell the Lineage: Genes Downstream of DMRT1
7. Chicken Sertoli Cell Differentiation Revealed by Single Cell RNA-Seq
8. Interstitial and Steroidogenic Cell Differentiation
9. Sex Steroid Synthesis and Testis Development
10. Germ Cells in the Embryonic Chicken Testis
11. Interstitial Cells of the Embryonic Chicken Testis
12. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Doran, T.J.; Morris, K.R.; Wise, T.G.; O’Neil, T.E.; Cooper, C.A.; Jenkins, K.A.; Tizard, M.L.V. Sex selection in layer chickens. Anim. Prod. Sci. 2018, 58, 476. [Google Scholar] [CrossRef]
- Krautwald-Junghanns, M.-E.; Cramer, K.; Fischer, B.; Förster, A.; Galli, R.; Kremer, F.; Mapesa, E.U.; Meissner, S.; Preisinger, R.; Preusse, G.; et al. Current approaches to avoid the culling of day-old male chicks in the layer industry, with special reference to spectroscopic methods. Poult. Sci. 2018, 97, 749–757. [Google Scholar] [CrossRef]
- Chue, J.; Smith, C.A. Sex determination and sexual differentiation in the avian model. FEBS J. 2011, 278, 1027–1034. [Google Scholar] [CrossRef]
- Major, A.; Ayers, K.L.; Chue, J.; Roeszler, K.N.; A Smith, C. FOXL2 antagonises the male developmental pathway in embryonic chicken gonads. J. Endocrinol. 2019, 243, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Sekido, R.; Lovell-Badge, R. Mechanisms of gonadal morphogenesis are not conserved between chick and mouse. Dev. Biol. 2007, 302, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Guioli, S.; Zhao, D.; Nandi, S.; Clinton, M.; Lovell-Badge, R. Oestrogen in the chick embryo can induce chromosomally male ZZ left gonad epithelial cells to form an ovarian cortex that can support oogenesis. Development 2020, 147, dev181693. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Murai, H.; Saito, D. Hedgehog–BMP signalling establishes dorsoventral patterning in lateral plate mesoderm to trigger gonadogenesis in chicken embryos. Nat. Commun. 2016, 7, 12561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, C.E.; Serralbo, O.; Ayers, K.L.; Roeszler, K.N.; Smith, C.A. Genetic manipulation of the avian urogenital system using in ovo electroporation. Avian Reptil. Dev. Biol. 2017, 1650, 177–190. [Google Scholar] [CrossRef]
- Nanda, I.; Schlegelmilch, K.; Haaf, T.; Schartl, M.; Schmid, M. Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenet. Genome Res. 2008, 122, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Handley, L.L.; Ceplitis, H.; Ellegren, H. Evolutionary strata on the chicken z chromosome: Implications for sex chromosome evolution. Genetics 2004, 167, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Fridolfsson, A.-K.; Cheng, H.; Copeland, N.G.; Jenkins, N.A.; Liu, H.-C.; Raudsepp, T.; Woodage, T.; Chowdhary, B.; Halverson, J.; Ellegren, H. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc. Natl. Acad. Sci. USA 1998, 95, 8147–8152. [Google Scholar] [CrossRef] [Green Version]
- Ayers, K.L.; Davidson, N.M.; Demiyah, D.; Roeszler, K.N.; Grützner, F.; Sinclair, A.H.; Oshlack, A.; Smith, C.A. RNA sequencing reveals sexually dimorphic gene ex-pression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome. Genome Biol. 2013, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhou, Q. The female-specific W chromosomes of birds have conserved gene contents but are not feminized. Genes 2020, 11, 1126. [Google Scholar] [CrossRef]
- Smeds, L.; Warmuth, V.; Bolivar, P.; Uebbing, S.; Burri, R.; Suh, A.; Nater, A.; Bureš, S.; Garamszegi, L.Z.; Hogner, S.; et al. Evolutionary analysis of the female-specific avian W chromosome. Nat. Commun. 2015, 6, 7330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; McBride, D.; Nandi, S.; McQueen, H.A.; McGrew, M.J.; Hocking, P.M.; Lewis, P.D.; Sang, H.M.; Clinton, M. Somatic sex identity is cell autonomous in the chicken. Nature 2010, 464, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, A.; Smith, C.A. Sex reversal in birds. Sex. Dev. 2016, 10, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Hirst, C.E.; Major, A.T.; Ezaz, T.; Ford, M.; Bibby, S.; Doran, T.J.; Smith, C.A. Gonadal and endocrine analysis of a gynandromorphic chicken. Endocrinology 2018, 159, 3492–3502. [Google Scholar] [CrossRef]
- Clinton, M.; Zhao, D.; Nandi, S.; McBride, D. Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Res. 2012, 20, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Scheib, D. Effects and role of estrogens in avian gonadal differentiation. Mech. Gonadal Differ. Vertebr. 1983, 87–92. [Google Scholar] [CrossRef]
- Nishikimi, H.; Kansaku, N.; Saito, N.; Usami, M.; Ohno, Y.; Shimada, K. Sex differentiation and mRNA expression of P450c17, P450arom and AMH in gonads of the chicken. Mol. Reprod Dev. 2000, 55, 20–30. [Google Scholar] [CrossRef]
- Andrews, J.E.; Smith, C.A.; Sinclair, A.H. Sites of estrogen receptor and aromatase expression in the chicken embryo. Gen. Comp. Endocr. 1997, 108, 182–190. [Google Scholar] [CrossRef]
- Elbrecht, A.; Smith, R. Aromatase enzyme activity and sex determination in chickens. Science 1992, 255, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, L.S.; Cummins, D.M.; Doran, T.J.; Sinclair, A.H.; Smith, C.A. Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos. PLoS ONE 2013, 8, e68362. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.O.; Mathur, S.; Hattem, G.; Tassy, O.; Pourquié, O. Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos. BMC Genom. 2010, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Graves, J.A.M. Avian sex, sex chromosomes, and dosage compensation in the age of genomics. Chromosom. Res. 2014, 22, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Uebbing, S.; Künstner, A.; Mäkinen, H.; Ellegren, H. Transcriptome sequencing reveals the character of incomplete dosage compensation across multiple tissues in flycatchers. Genome Biol. Evol. 2013, 5, 1555–1566. [Google Scholar] [CrossRef] [Green Version]
- Adolfsson, S.; Ellegren, H. Lack of dosage compensation accompanies the arrested stage of sex chromosome evolution in ostriches. Mol. Biol. Evol. 2013, 30, 806–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birchler, J.A. Dosage compensation for the birds. Heredity 2009, 102, 423–424. [Google Scholar] [CrossRef]
- Melamed, E.; Arnold, A.P. Regional differences in dosage compensation on the chicken Z chromosome. Genome Biol. 2007, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, Y.; Melamed, E.; Yang, X.; Kampf, K.; Wang, S.; Yehya, N.; Van Nas, A.; Replogle, K.; Band, M.R.; Clayton, D.F.; et al. Dosage compensation is less effective in birds than in mammals. J. Biol. 2007, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellegren, H.; Hultin-Rosenberg, L.; Brunström, B.; Dencker, L.; Kultima, K.; Scholz, B. Faced with inequality: Chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.A.; Major, A.T.; Estermann, M.A. The curious case of avian sex determination. Trends Genet. 2021, 37, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Leska, A.; Dusza, L. Seasonal changes in the hypothalamo-pituitary-gonadal axis in birds. Reprod. Biol. 2007, 7. [Google Scholar]
- Kirby, J.F.D. Reproduction in Male Birds. Sturkie’s Avian Physiology, 5th ed; Academic Press: Main St. Salt Lake City, UT, USA, 1999; pp. 597–615. [Google Scholar]
- McCartney, M.G. Sexual maturity in broiler breeder males. Poult. Sci. 1978, 57, 1720–1722. [Google Scholar] [CrossRef]
- Cooksey, E.J.; Rothwell, B. The ultrastructure of the Sertoli cell and its differentiation in the domestic fowl (Gallus domesticus). J. Anat. 1973, 114, 329–345. [Google Scholar] [PubMed]
- Ishii, S.; Furuya, T. Effects of purified chicken gonadotropins on the chick testis. Gen. Comp. Endocrinol. 1975, 25, 1–8. [Google Scholar] [CrossRef]
- Brown, N.; Follett, B. Effects of androgens on the testes of intact and hypophysectomized Japanese quail. Gen. Comp. Endocrinol. 1977, 33, 267–277. [Google Scholar] [CrossRef]
- Cooper, C.A.; Challagulla, A.; Jenkins, K.A.; Wise, T.G.; O′Neil, T.; Morris, K.; Tizard, M.; Doran, T.J. Generation of gene edited birds in one generation using sperm transfection assisted gene editing (STAGE). Transgenic Res. 2016, 26, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.; Brière, S.; Pelletier, R.; Brillard, J.P.; Froment, P. Characterization of chicken Sertoli cells in vitro. Poult. Sci. 2011, 90, 1276–1286. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kagami, H.; Tagami, T. Development, differentiation and manipulation of chicken germ cells. Dev. Growth Differ. 2013, 55, 20–40. [Google Scholar] [CrossRef]
- Ballantyne, M.; Woodcock, M.; Doddamani, D.; Hu, T.; Taylor, L.; Hawken, R.J.; McGrew, M.J. Direct allele introgression into pure chicken breeds using Sire Dam Surrogate (SDS) mating. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, M.E.; Gheyas, A.A.; Mason, A.; Nandi, S.; Taylor, L.; Sherman, A.; Smith, J.; Burt, D.W.; Hawken, R.; McGrew, M.J. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc. Natl. Acad. Sci. USA 2019, 116, 20930–20937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucksova, J.; Reinisova, M.; Kalina, J.; Lejckova, B.; Hejnar, J.; Trefil, P. Conservation of chicken male germline by orthotopic trans-plantation of primordial germ cells from genetically distant donorsdagger. Biol. Reprod. 2019, 101, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Jeong, D.K.; Kim, J.N.; Song, G.H.; Hong, Y.H.; Lim, J.M.; Han, J.Y. Improved germline transmission in chicken chimeras produced by trans-plantation of gonadal primordial germ cells into recipient embryos. Biol. Reprod. 2003, 68, 1657–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambeth, L.S.; Ayers, K.L.; Cutting, A.; Doran, T.J.; Sinclair, A.; Smith, C. Anti-müllerian hormone is required for chicken embryonic urogenital system growth but not sexual differentiation. Biol. Reprod. 2015, 93, 138. [Google Scholar] [CrossRef]
- Smith, C.A.; Major, A.T.; Estermann, M.A. Chickens, sex, and revisiting an old paradigm. Endocrinology 2021, 162, bqab106. [Google Scholar] [CrossRef]
- Mi, Y.; He, B.; Li, J.; Zhang, C. Progesterone regulates chicken embryonic germ cell meiotic initiation independent of retinoic acid signaling. Theriogenology 2014, 82, 195–203. [Google Scholar] [CrossRef]
- Guioli, S.; Nandi, S.; Zhao, D.; Burgess-Shannon, J.; Lovell-Badge, R.; Clinton, M. Gonadal Asymmetry and Sex Determination in Birds. Sex. Dev. 2014, 8, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Carlon, N.; Stahl, A. Origin of the somatic components in chick embryonic gonads. Arch. D’anatomie Microsc. Morphol. Exp. 1985, 74, 52–59. [Google Scholar]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Saito, D. Epithelial-to-mesenchymal transition–based morphogenesis of dorsal mesentery and gonad. Semin. Cell Dev. Biol. 2018, 92, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ebensperger, C.; Drews, U.; Mayerová, A.; Wolf, U. Serological HY antigen in the female chicken occurs during gonadal differentiation. Differentiation 1988, 37, 186–191. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ishimaru, Y.; Sugiyama, N.; Tsunekawa, N.; Noce, T.; Kasahara, M.; Morohashi, K.I. Mesonephric FGF signaling is associated with the de-velopment of sexually indifferent gonadal primordium in chick embryos. Dev. Biol. 2005, 280, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Oréal, E.; Mazaud, S.; Picard, J.Y.; Magre, S.; Carré-Eusèbe, D. Different patterns of anti-Mullerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev. Dynam. 2002, 225, 221–232. [Google Scholar] [CrossRef]
- Colvin, J.S.; Green, R.P.; Schmahl, J.; Capel, B.; Ornitz, D. Male-to-female sex reversal in mice lacking fibroblast growth factor. Cell 2001, 104, 875–889. [Google Scholar] [CrossRef]
- Kim, Y.; Kobayashi, A.; Sekido, R.; DiNapoli, L.; Brennan, J.; Chaboissier, M.-C.; Poulat, F.; Behringer, R.R.; Lovell-Badge, R.; Capel, B. FGF9 and Wnt4 Act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006, 4, e187. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Ikeda, Y.; Parker, K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994, 77, 481–490. [Google Scholar] [CrossRef]
- Ayers, K.L.; Smith, C.; Lambeth, L.S. The molecular genetics of avian sex determination and its manipulation. Genesis 2013, 51, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Hirst, C.; Major, A.; Ayers, K.L.; Brown, R.J.; Mariette, M.; Sackton, T.; Smith, C. Sex reversal and comparative data undermine the W chromosome and support Z-linked DMRT1 as the regulator of gonadal sex differentiation in birds. Endocrinology 2017, 158, 2970–2987. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Sinclair, A.H. Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. Int. J. Dev. Biol. 2009, 53, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bellott, D.W.; Page, D.C. Dosage-sensitive functions in embryonic development drove the survival of genes on sex-specific chromosomes in snakes, birds, and mammals. Genome Res. 2021, 31, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Bellott, D.W.; Skaletsky, H.; Cho, T.-J.; Brown, L.; Locke, D.; Chen, N.; Galkina, S.; Pyntikova, T.; Koutseva, N.; Graves, T.; et al. Avian W and mammalian Y chromosomes convergently retained dosage-sensitive regulators. Nat. Genet. 2017, 49, 387–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellott, D.W.; Skaletsky, H.; Pyntikova, T.; Mardis, E.R.; Graves, T.; Kremitzki, C.; Brown, L.G.; Rozen, S.G.; Warren, W.C.; Wilson, R.K.; et al. Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition. Nature 2010, 466, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Ezaz, T.; Stiglec, R.; Veyrunes, F.; Graves, J. Relationships between vertebrate ZW and XY sex chromosome systems. Curr. Biol. 2006, 16, R736–R743. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.; Sinclair, A. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef]
- Nakata, T.; Ishiguro, M.; Aduma, N.; Izumi, H.; Kuroiwa, A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 3417–3422. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A.; Carlon, N. Morphogenesis of the sex cords and the significance of the medullary zone of the gonad in the chick embryo. Acta Anatom 1973, 85, 248–274. [Google Scholar] [CrossRef]
- Van Limborgh, J. The first sign of sexual differentiation of the gonads in chick embryos. Arch. D’anatomie Microsc. Morphol. Exp. 1968, 57, 79–90. [Google Scholar]
- Tsunekawa, N.; Naito, M.; Sakai, Y.; Nishida, T.; Noce, T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000, 127, 2741–2750. [Google Scholar] [CrossRef]
- Smith, C.A.; Sinclair, A. Sex determination: Insights from the chicken. BioEssays 2004, 26, 120–132. [Google Scholar] [CrossRef]
- Zaccanti, F.; Vallisneri, M.; Quaglia, A. Early aspects of sex differentiation in the gonads of chick embryos. Differentiation 1990, 43, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Nagano, R.; Kanai, Y.; Kurohmaru, M.; Hayashi, Y.; Nishida, T. Distribution of desmin and fibronectin in chick embryo gonad during testicular cord formation. J. Veter- Med. Sci. 1997, 59, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Lambeth, L.S.; Raymond, C.; Roeszler, K.N.; Kuroiwa, A.; Nakata, T.; Zarkower, D.; Smith, C.A. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014, 389, 160–172. [Google Scholar] [CrossRef] [Green Version]
- DeFalco, T.; Takahashi, S.; Capel, B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev. Biol. 2011, 352, 14–26. [Google Scholar] [CrossRef]
- Combes, A.; Wilhelm, D.; Davidson, T.; Dejana, E.; Harley, V.; Sinclair, A.; Koopman, P. Endothelial cell migration directs testis cord formation. Dev. Biol. 2009, 326, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Rotgers, E.; Jørgensen, A.; Yao, H.H.-C. At the crossroads of fate—Somatic cell lineage specification in the fetal gonad. Endocr. Rev. 2018, 39, 739–759. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.; Capel, B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev. Biol. 1998, 203, 323–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stévant, I.; Kühne, F.; Greenfield, A.; Chaboissier, M.C.; Dermitzakis, E.T.; Nef, S. Dissecting cell lineage specification and sex fate determination in gonadal somatic cells using single-cell transcriptomics. Cell Rep. 2019, 26, 3272–3283. [Google Scholar] [CrossRef] [Green Version]
- Stévant, I.; Neirijnck, Y.; Borel, C.; Escoffier, J.; Smith, L.B.; Antonarakis, S.E.; Dermitzakis, E.T.; Nef, S. Deciphering cell lineage specification during male sex determination with single-cell RNA sequencing. Cell Rep. 2018, 22, 1589–1599. [Google Scholar] [CrossRef] [Green Version]
- Merchant-Larios, H.; Popova, L.; Reyss-Brion, M. Early morphogenesis of chick gonad in the absence of mesonephros. (morphogenesis/gonad/mesonephric agenesis). Dev. Growth Differ. 1984, 26, 403–417. [Google Scholar] [CrossRef]
- Rodemer, E.S.; Ihmer, A.; Wartenberg, H. Gonadal development of the chick embryo following microsurgically caused agenesis of the mesonephros and using interspecific quail-chick chimaeras. J. Embryol. Exp. Morphol. 1986, 98, 269–285. [Google Scholar]
- Hirst, C.E.; Major, A.T.; Smith, C.A. Sex determination and gonadal sex differentiation in the chicken model. Int. J. Dev. Biol. 2018, 62, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Roeszler, K.N.; Hudson, Q.J.; Sinclair, A.H. Avian sex determination: What, when and where? Cytogenet. Genome Res. 2007, 117, 165–173. [Google Scholar] [CrossRef]
- Estermann, M.A.; Williams, S.; Hirst, C.E.; Roly, Z.Y.; Serralbo, O.; Adhikari, D.; Powell, D.; Major, A.; Smith, C.A. Insights into gonadal sex differentiation provided by single-cell transcriptomics in the chicken embryo. Cell Rep. 2020, 31, 107491. [Google Scholar] [CrossRef] [PubMed]
- Tena, J.J.; Neto, A.; de la Calle-Mustienes, E.; Pereira, C.A.B.S.; Casares, F.; Gómez-Skarmeta, J.L. Odd-skipped genes encode repressors that control kidney development. Dev. Biol. 2007, 301, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lan, Y.; Cho, E.S.; Maltby, K.M.; Jiang, R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and uro-genital development. Dev. Biol. 2005, 288, 582–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, R.; Kamei, C.N.; Wang, Q.; Jiang, R.; Schultheiss, T.M. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 2006, 133, 2995–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.A.; Shoemaker, C.M.; Roeszler, K.N.; Queen, J.; Crews, D.; Sinclair, A.H. Cloning and expression of R-Spondin1in different vertebrates suggests a conserved role in ovarian development. BMC Dev. Biol. 2008, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Matson, C.K.; Zarkower, D. Sex and the singular DM domain: Insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 2012, 13, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Zarkower, D. DMRT genes in vertebrate gametogenesis. Curr. Top. Dev. Biol. 2013, 102, 327–356. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.S.; Murphy, M.W.; O′Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. DMRT1, a gene related to worm and fly sexual regu-lators, is required for mammalian testis differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [CrossRef] [Green Version]
- Koopman, P. Sex determination: The power of DMRT1. Trends Genet. 2009, 25, 479–481. [Google Scholar] [CrossRef]
- Kim, S.; Bardwell, V.J.; Zarkower, D. Cell type-autonomous and non-autonomous requirements for DMRT1 in postnatal testis differentiation. Dev. Biol. 2007, 307, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011, 476, 101–104. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Huang, S. Sex determination and maintenance: The role of DMRT1 and FOXL2. Asian J. Androl. 2017, 19, 619–624. [Google Scholar] [CrossRef]
- Zhang, T.; Oatley, J.; Bardwell, V.J.; Zarkower, D. DMRT1 Is Required for mouse spermatogonial stem cell maintenance and replenishment. PLoS Genet. 2016, 12, e1006293. [Google Scholar] [CrossRef] [Green Version]
- Lindeman, R.E.; Gearhart, M.; Minkina, A.; Krentz, A.D.; Bardwell, V.J.; Zarkower, D. Sexual cell-fate reprogramming in the ovary by DMRT1. Curr. Biol. 2015, 25, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Cai, H.; Zhang, G.; Zhang, H.; Bao, H.; Wang, L.; Ye, J.; Qian, G.; Ge, C. DMRT1 is required for primary male sexual differentiation in Chinese soft-shelled turtle Pelodiscus sinensis. Sci. Rep. 2017, 7, 4433. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Ye, J.; Zhang, H.; Zhang, Y.; Sun, W.; Sang, Y.; Capel, B.; Qian, G. DMRT1 induces the male pathway in a turtle species with temperature-dependent sex determination. Development 2017, 144, 2222–2233. [Google Scholar] [PubMed] [Green Version]
- Shoemaker, C.; Ramsey, M.; Queen, J.; Crews, D. Expression of Sox9, Mis, and DMRT1 in the gonad of a species with temperature-dependent sex determination. Dev. Dynam. 2007, 236, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, Y.; Wang, W.; Wang, Q.; Zhang, N.; Lin, F.; Wang, N.; Shao, C.; Dong, Z.; Li, Y.; et al. Genome editing reveals DMRT1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis). Sci. Rep. 2017, 7, 42213. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Yamada, M.; Kamei, Y.; Fujiwara-Ishikawa, T.; Todo, T.; Nagahama, Y.; Matsuda, M. DMRT1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosom. Res. 2011, 20, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Matsuda, M.; Kajiura-Kobayashi, H.; Suzuki, A.; Saito, N.; Nakamoto, M.; Shibata, N.; Nagahama, Y. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev. Dyn. 2004, 231, 518–526. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male devel-opment in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Mawaribuchi, S.; Musashijima, M.; Wada, M.; Izutsu, Y.; Kurakata, E.; Park, M.K.; Takamatsu, N.; Ito, M. Molecular Evolution of Two Distinct DMRT1 Promoters for Germ and Somatic Cells in Vertebrate Gonads. Mol. Biol. Evol. 2016, 34, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Schach, U.; Ordaz, A.; Steinfeld, J.S.; Draper, B.W.; Siegfried, K.R. DMRT1 is necessary for male sexual development in zebrafish. Dev. Biol. 2016, 422, 33–46. [Google Scholar] [CrossRef]
- Nanda, I.; Zend-Ajusch, E.; Shan, Z.; Grützner, F.; Schartl, M.; Burt, D.; Koehler, M.; Fowler, V.; Goodwin, G.; Schneider, W.; et al. Conserved synteny between the chicken Z sex chromosome and human chromosome 9 includes the male regulatory gene DMRT1: A comparative (re)view on avian sex determination. Cytogenet. Genome Res. 2000, 89, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Kirby, P.; Zarkower, D.; Graves, J.A. DMRT1 in a ratite bird: Evidence for a role in sex determination and discovery of a putative regulatory element. Cytogenet. Genome Res. 2002, 99, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, J.; Bachtrog, D.; An, N.; Huang, Q.; Jarvis, E.D.; Gilbert, M.; Zhang, G. Complex evolutionary trajectories of sex chromosomes across bird taxa. Science 2014, 346, 1246338. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Nanda, I.; Wang, Y.; Schmid, M.; Vortkamp, A.; Haaf, T. Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds. Cytogenet. Cell Genet. 2000, 89, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.S.; Kettlewell, J.R.; Hirsch, B.; Bardwell, V.J.; Zarkower, D. Expression of DMRT1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev. Biol. 1999, 215, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; McClive, P.J.; Western, P.; Reed, K.J.; Sinclair, A. Conservation of a sex-determining gene. Nature 1999, 402, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, M.; Choi, H.J.; Rengaraj, D.; Jung, K.M.; Park, J.S.; Lee, K.Y.; Kim, Y.M.; Park, K.J.; Han, S.T.; et al. DMRT1 gene disruption alone induces incomplete gonad feminization in chicken. FASEB J. 2021, 35, e21876. [Google Scholar] [CrossRef]
- Bai, D.-P.; Chen, Y.; Hu, Y.-Q.; He, W.-F.; Shi, Y.-Z.; Fan, Q.-M.; Luo, R.-T.; Li, A. Transcriptome analysis of genes related to gonad differentiation and development in Muscovy ducks. BMC Genom. 2020, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Naurin, S.; Hasselquist, D.; Bensch, S.; Hansson, B. Sex-biased gene expression on the avian Z chromosome: Highly ex-pressed genes show higher male-biased expression. PLoS ONE 2012, 7, e46854. [Google Scholar] [CrossRef]
- Wright, A.E.; Moghadam, H.; Mank, J. Trade-off between selection for dosage compensation and masculinization on the avian Z chromosome. Genetics 2012, 192, 1433–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omotehara, T.; Smith, C.; Mantani, Y.; Kobayashi, Y.; Tatsumi, A.; Nagahara, D.; Hashimoto, R.; Hirano, T.; Umemura, Y.; Yokoyama, T.; et al. Spatiotemporal expression patterns of doublesex and mab-3 related transcription factor 1 in the chicken developing gonads and Müllerian ducts. Poult. Sci. 2014, 93, 953–958. [Google Scholar] [CrossRef]
- Lei, N.; Hornbaker, K.I.; Rice, D.A.; Karpova, T.; Agbor, V.A.; Heckert, L.L. Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development. Biol. Reprod. 2007, 77, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Krentz, A.D.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev. Biol. 2011, 356, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Kanatsu-Shinohara, M.; Hirose, M.; Ogura, A.; Shinohara, T. Pluripotent cell derivation from male germline cells by suppression of DMRT1 and Trp53. J. Reprod. Dev. 2015, 61, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krentz, A.D.; Murphy, M.W.; Kim, S.; Cook, M.S.; Capel, B.; Zhu, R.; Matin, A.; Sarver, A.L.; Parker, K.L.; Griswold, M.D.; et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl. Acad. Sci. USA 2009, 106, 22323–22328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The mammalian doublesex homolog DMRT1 is a tran-scriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrish, B.C.; Sinclair, A.H. Vertebrate sex determination: Many means to an end. Reproduction 2002, 124, 447–457. [Google Scholar] [CrossRef]
- Sekido, R.; Bar, I.; Narváez, V.; Penny, G.; Lovell-Badge, R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev. Biol. 2004, 274, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekido, R.; Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008, 453, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Gonen, N.; Lovell-Badge, R. The regulation of Sox9 expression in the gonad. Curr. Top. Dev. Biol. 2019, 134, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Croft, B.; Ohnesorg, T.; Hewitt, J.; Bowles, J.; Quinn, A.; Tan, J.; Corbin, V.; Pelosi, E.; Bergen, J.V.D.; Sreenivasan, R.; et al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gonen, N.; Quinn, A.; O’Neill, H.C.; Koopman, P.; Lovell-Badge, R. Normal levels of Sox9 expression in the developing mouse testis depend on the TES/TESCO enhancer, but this does not act alone. PLoS Genet. 2017, 13, e1006520. [Google Scholar]
- Takada, S.; Ota, J.; Kansaku, N.; Yamashita, H.; Izumi, T.; Ishikawa, M.; Wada, T.; Kaneda, R.; Choi, Y.L.; Koinuma, K.; et al. Nucleotide sequence and embryonic expression of quail and duck Sox9 genes. Gen. Comp. Endocrinol. 2006, 145, 208–213. [Google Scholar] [CrossRef]
- Kent, J.; Wheatley, S.C.; Andrews, J.E.; Sinclair, A.; Koopman, P. A male-specific role for SOX9 in vertebrate sex determination. Development 1996, 122, 2813–2822. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Hacker, A.; Harley, V.; Goodfellow, P.; Swain, A.; Lovell-Badge, R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 1996, 14, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.P.; Chaboissier, M.-C.; de Rooij, D.; Schedl, A. Sox9 induces testis development in XX transgenic mice. Nat. Genet. 2001, 28, 216–217. [Google Scholar] [CrossRef] [PubMed]
- De Santa Barbara, P.; Bonneaud, N.; Boizet, B.; Desclozeaux, M.; Moniot, B.; Sudbeck, P.; Scherer, G.; Poulat, F.; Berta, P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol. Cell Biol. 1998, 18, 6653–6665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oreal, E.; Pieau, C.; Mattei, M.-G.; Josso, N.; Picard, J.-Y.; Carré-Eusèbe, D.; Magre, S. Early expression ofAMH in chicken embryonic gonads precedes testicularSOX9 expression. Dev. Dyn. 1998, 212, 522–532. [Google Scholar] [CrossRef]
- Smith, C.; Smith, M.J.; Sinclair, A. Gene expression during gonadogenesis in the chicken embryo. Gene 1999, 234, 395–402. [Google Scholar] [CrossRef]
- Wilhelm, D.; Hiramatsu, R.; Mizusaki, H.; Widjaja, L.; Combes, A.; Kanai, Y.; Koopman, P. SOX9 Regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J. Biol. Chem. 2007, 282, 10553–10560. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, D.; Martinson, F.; Bradford, S.; Wilson, M.J.; Combes, A.N.; Beverdam, A.; Bowles, J.; Mizusaki, H.; Koopman, P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev. Biol. 2005, 287, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Moniot, B.; Declosmenil, F.; Barrionuevo, F.; Scherer, G.; Aritake, K.; Malki, S.; Marzi, L.; Cohen-Solal, A.; Georg, I.; Klattig, J.; et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 2009, 136, 1813–1821. [Google Scholar] [CrossRef] [Green Version]
- Moniot, B.; Boizet-Bonhoure, B.; Poulat, F. Male specific expression of lipocalin-type prostaglandin D synthase (cPTGDS) during chicken gonadal differentiation: Relationship with cSOX9. Sex. Dev. 2008, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Willerton, L.; Smith, R.A.; Russell, D.; Mackay, S. Effects of FGF9 on embryonic Sertoli cell proliferation and testicular cord formation in the mouse. Int. J. Dev. Biol. 2004, 48, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Fam, S.; Sim, H.; Bernard, P.; Jayakody, I.; Taketo, M.M.; Scherer, G.; Harley, V. Loss of Fgfr2 leads to partial XY sex reversal. Dev. Biol. 2008, 314, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Bingham, N.; Sekido, R.; Parker, K.L.; Lovell-Badge, R.; Capel, B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc. Natl. Acad. Sci. USA 2007, 104, 16558–16563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmahl, J.; Kim, Y.; Colvin, J.S.; Ornitz, D.M.; Capel, B. FGF9induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 2004, 131, 3627–3636. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Kataoka, K.; Yamamoto, H.; Kato, T.; Hara, S.; Yamaguchi, K.; Renard-Guillet, C.; Katou, Y.; Shirahige, K.; Ochi, H.; et al. Comparative analysis demonstrates cell type-specific conservation of SOX9 targets between mouse and chicken. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rahmoun, M.; Lavery, R.; Laurent-Chaballier, S.; Bellora, N.; Philip, G.K.; Rossitto, M.; Symon, A.; Pailhoux, E.; Cammas, F.; Chung, J.; et al. In mammalian foetal testes, SOX9 regulates expression of its target genes by binding to genomic regions with conserved signatures. Nucleic Acids Res. 2017, 45, 7191–7211. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.W.; Sarver, A.L.; Rice, D.; Hatzi, K.; Ye, K.; Melnick, A.; Heckert, L.L.; Zarkower, D.; Bardwell, V.J. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc. Natl. Acad. Sci. USA 2010, 107, 13360–13365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.W.; Zarkower, D.; Bardwell, V.J. Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. BMC Mol. Biol. 2007, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Nicholson, R.H.; Kaplan, J.; Galy, A.; Li, L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech. Dev. 2001, 104, 105–111. [Google Scholar] [CrossRef]
- Zhang, H.; Menzies, K.J.; Auwerx, J. The role of mitochondria in stem cell fate and aging. Development 2018, 145, dev143420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, D.; Palmer, S.; Koopman, P. Sex Determination and gonadal development in mammals. Physiol. Rev. 2007, 87, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Windley, S.; Wilhelm, D. Signaling pathways involved in mammalian sex determination and gonad development. Sex. Dev. 2016, 9, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Franco, H.L.; Yao, H.H. Sex and hedgehog: Roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res. 2012, 20, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Barsoum, I.; Yao, H.H. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal leydig cells. Biol. Reprod. 2011, 84, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitgood, M.J.; Shen, L.; McMahon, A.P. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 1996, 6, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Tong, M.; Jameson, J. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult leydig cell development. Endocrinology 2007, 148, 3704–3710. [Google Scholar] [CrossRef]
- Brennan, J.; Tilmann, C.; Capel, B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003, 17, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.; McClive, P.J.; Hudson, Q.; Sinclair, A.H. Male-specific cell migration into the developing gonad is a conserved process involving PDGF signalling. Dev. Biol. 2005, 284, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Jeays-Ward, K.; Hoyle, C.; Brennan, J.; Dandonneau, M.; Alldus, G.; Capel, B.; Swain, A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 2003, 130, 3663–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.-C.H.; Matzuk, M.M.; Jorgez, C.J.; Menke, D.; Page, D.C.; Swain, A.; Capel, B. Follistatin operates downstream ofWnt4 in mammalian ovary organogenesis. Dev. Dyn. 2004, 230, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommes, R.C. Vasculogenesis in Selected Endocrine Glnads of Normal and Hypothesectomized Chick Embryos. PhD Thesis, Northwestern University, Evanston, IL, USA; pp. 1–500.
- Gonzalez-Moran, M.G.; Soria-Castro, E. Histological and stereological studies on Leydig cells in the testes of Gallus do-mesticus from pre-hatching to sexual maturity. Anim. Reprod. Sci. 2010, 120, 129–135. [Google Scholar] [CrossRef]
- Haffen, K. Sex differentiation of avian gonads in vitro. Am. Zoöl. 1975, 15, 257–272. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, B.; Nabipour, A.; Rassouli, M.B.; Dehghani, H. Morphological development of testes in ostrich (Struthio camelus) embryo. Anat. Sci. Int. 2013, 89, 129–139. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Cui, X.; Lin, X.; Li, Y.; Wang, Y.; Wang, Y.; Qin, Y.; Chen, D.; Han, C.; et al. Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development 2016, 144, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Chen, M.; Wen, Q.; Li, Y.; Wang, Y.; Wang, Y.; Qin, Y.; Cui, X.; Yang, L.; Huff, V.; et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc. Natl. Acad. Sci. USA 2015, 112, 4003–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchant-Larios, H.; Moreno-Mendoza, N. Mesonephric stromal cells differentiate into leydig cells in the mouse fetal testis. Exp. Cell Res. 1998, 244, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Estermann, M.A.; Major, A.T.; Smith, C.A. Gonadal sex differentiation: Supporting versus steroidogenic cell lineage specification in mammals and birds. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Lambeth, L.S.; Morris, K.; Ayers, K.L.; Wise, T.G.; O′Neil, T.; Wilson, S.; Cao, Y.; Sinclair, A.H.; Cutting, A.D.; Doran, T.J.; et al. Overexpression of anti-mullerian hormone disrupts gonadal sex differentiation, blocks sex hormone synthesis, and supports cell autonomous sex development in the chicken. Endocrinology 2016, 157, 1258–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archambeault, D.R.; Yao, H.-C.H. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 10526–10531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaillant, S.; Dorizzi, M.; Pieau, C.; Richard-Mercier, N. Sex reversal and aromatase in chicken. J. Exp. Zoöl. 2001, 290, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Ayers, K.; Sinclair, A.; Smith, C. The molecular genetics of ovarian differentiation in the avian model. Sex. Dev. 2013, 7, 80–94. [Google Scholar] [CrossRef]
- Woods, J.E.; Podczaski, E.S. Androgen synthesis in the gonads of the chick embryo. Gen. Comp. Endocrinol. 1974, 24, 413–423. [Google Scholar] [CrossRef]
- Groenendijk-Huijbers, M.M.; Van Schaik, J.P. Effects of hemicastration, testis implantation and administration of tes-tosterone propionate on the female embryonic genital tract in various breeds and strains of chickens. Verh. Anat. Ges. 1976, 70, 179–182. [Google Scholar]
- Woods, J.E.; Simpson, R.M.; Moore, P.L. Plasma testosterone levels in the chick embryo. Gen. Comp. Endocrinol. 1975, 27, 543–547. [Google Scholar] [CrossRef]
- Weissmann, A.; Reitemeier, S.; Hahn, A.; Gottschalk, J.; Einspanier, A. Sexing domestic chicken before hatch: A new method for in ovo gender identification. Theriogenology 2013, 80, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Saito, N.; Nakamura, T. Ontogenetic steroidogenesis by testes, ovary, and adrenals of embryonic and postembryonic chickens (Gallus domesticus). Gen. Comp. Endocrinol. 1986, 63, 456–463. [Google Scholar] [CrossRef]
- Woods, J.E.; Thommes, R.C. Ontogeny of hypothalamo-adenohypophyseal-gonadal (HAG) interrelationships in the chick embryo. J. Exp. Zoöl. 1984, 232, 435–441. [Google Scholar] [CrossRef]
- Woods, J.E.; Podczaski, E.S.; Erton, L.H.; Rutherford, J.E.; McCarter, C.F. Establishment of the adenohypophyseal-testicular axis in the chick embryo I. Testicular androgen levels. Gen. Comp. Endocrinol. 1977, 32, 390–394. [Google Scholar] [CrossRef]
- Woods, J.E.; Honan, M.P.; Thommes, R.C. Hypothalamic regulation of the adenohypophyseal-testicular axis in the male chick embryo. Gen. Comp. Endocrinol. 1989, 74, 167–172. [Google Scholar] [CrossRef]
- Woods, J.E.; Weeks, R.L. Ontogenesis of the pituitary-gonadal axis in the chick embryo. Gen. Comp. Endocrinol. 1969, 13, 242–254. [Google Scholar] [CrossRef]
- Rios-Rojas, C.; Bowles, J.; Koopman, P. On the role of germ cells in mammalian gonad development: Quiet passengers or back-seat drivers? Reproduction 2015, 149, R181–R191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, C.; Koopman, P.; Bowles, J. Sex determination in the mammalian germline. Annu. Rev. Genet. 2017, 51, 265–285. [Google Scholar] [CrossRef]
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Koubova, J.; Menke, D.; Zhou, Q.; Capel, B.; Griswold, M.D.; Page, D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 2474–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernet, N.; Condrea, D.; Mayere, C.; Féret, B.; Klopfenstein, M.; Magnant, W.; Alunni, V.; Telentin, M.; Souali-Crespo, S.; Nef, S.; et al. Meiosis occurs normally in the fetal ovary of mice lacking all retinoic acid receptors. Sci. Adv. 2020, 6, eaaz1139. [Google Scholar] [CrossRef] [PubMed]
- Chassot, A.-A.; Le Rolle, M.; Jolivet, G.; Stevant, I.; Guigonis, J.-M.; Da Silva, F.; Nef, S.; Pailhoux, E.; Schedl, A.; Ghyselinck, N.B.; et al. Retinoic acid synthesis by ALDH1A proteins is dispensable for meiosis initiation in the mouse fetal ovary. Sci. Adv. 2020, 6, eaaz1261. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Roeszler, K.N.; Bowles, J.; Koopman, P.; Sinclair, A.H. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev. Biol. 2008, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bowles, J.; Feng, C.-W.; Ineson, J.; Miles, K.; Spiller, C.; Harley, V.; Sinclair, A.; Koopman, P. Retinoic acid antagonizes testis development in mice. Cell Rep. 2018, 24, 1330–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Q.; Jin, J.; Jin, K.; Sun, C.; Song, J.; Zhang, Y.; Chen, G.; Li, B. Distinct roles of retinoic acid and BMP4 pathways in the formation of chicken primordial germ cells and spermatogonial stem cells. Food Funct. 2019, 10, 7152–7163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Han, J.Y. The early development of germ cells in chicken. Int. J. Dev. Biol. 2018, 62, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Yu, P.; Leghari, I.H.; Ge, C.; Mi, Y.; Zhang, C. RALDH2, the enzyme for retinoic acid synthesis, mediates meiosis initiation in germ cells of the female embryonic chickens. Amino Acids 2012, 44, 405–412. [Google Scholar] [CrossRef]
- Yousefi Taemeh, S.; Mahdavi Shahri, N.; Lari, R.; Bahrami, A.R.; Dehghani, H. Meiotic initiation in chicken germ cells is regulated by Cyp26b1 and mesonephros. J. Exp. Zool. Part B 2019, 332, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ge, C.; Zeng, W.; Mi, Y.; Zhang, C. Retinoic acid promotes proliferation of chicken primordial germ cells via activation of PI3K/Akt-mediated NF-kappaB signalling cascade. Cell Biol. Int. 2012, 36, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lee, H.J.; Lee, H.C.; Hwang, Y.S.; Park, Y.H.; Ono, T.; Han, J.Y. The dynamic development of germ cells during chicken embryogenesis. Poult. Sci. 2018, 97, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, Y.; Usui, F.; Mushika, T.; Ono, T.; Setioko, A.R.; Takeda, K.; Nirasawa, K.; Kagami, H.; Tagami, T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult. Sci. 2007, 86, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S.H.; Meachem, S.J.; Sarraj, M.A.; Loveland, K.L. Activin a balances sertoli and germ cell proliferation in the fetal mouse testis. Biol. Reprod. 2010, 84, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Young, J.C.; Wakitani, S.; Loveland, K.L. TGF-beta superfamily signaling in testis formation and early male germline de-velopment. Semin. Cell Dev. Biol. 2015, 45, 94–103. [Google Scholar] [CrossRef]
- Tagami, T.; Miyahara, D.; Nakamura, Y. Avian primordial germ cells. Avian Reprod. 2017, 1001, 1–18. [Google Scholar] [CrossRef]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.; et al. A Comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-Seq. Dev. Cell 2018, 46, 651–667. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanes, A.; Wilhelm, D.; Wilson, M.J.; Bowles, J.; McClive, P.J.; Sinclair, A.H.; Koopman, P. Evaluation of candidate markers for the peritubular myoid cell lineage in the developing mouse testis. Reproduction 2005, 130, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Rebourcet, D.; O′Shaughnessy, P.J.; Pitetti, J.-L.; Monteiro, A.; O′Hara, L.; Milne, L.; Tsai, Y.T.; Cruickshanks, L.; Riethmacher, D.; Guillou, F.; et al. Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development 2014, 141, 2139–2149. [Google Scholar] [CrossRef] [Green Version]
- Lei, N.; Heckert, L.L. Gata4 Regulates Testis Expression of DMRT1. Mol. Cell. Biol. 2004, 24, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Lei, N.; Heckert, L.L. Sp1 and Egr1 Regulate Transcription of the DMRT1 Gene in Sertoli Cells1. Biol. Reprod. 2002, 66, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Ellis, H.L.; Shioda, K.; Rosenthal, N.F.; Coser, K.R.; Shioda, T. Masculine epigenetic sex marks of the CYP19A1/aromatase promoter in genetically male chicken embryonic gonads are resistant to estrogen-induced phenotypic sex conversion. Biol. Reprod. 2012, 87, 23. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Cai, C.; Qian, G.; Capel, B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 2018, 360, 645–648. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.G.; Balic, A.; Rainger, J.; Sang, H.M.; McGrew, M.J. Illuminating the chicken model through genetic modification. Int. J. Dev. Biol. 2018, 62, 257–264. [Google Scholar] [CrossRef]
- Macdonald, J.; Taylor, L.; Sherman, A.; Kawakami, K.; Takahashi, Y.; Sang, H.M.; McGrew, M.J. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc. Natl. Acad. Sci. USA 2012, 109, E1466–E1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrew, M.J.; Sherman, A.; Ellard, F.M.; Lillico, S.G.; Gilhooley, H.J.; Kingsman, A.J.; Mitrophanous, K.A.; Sang, H. Efficient production of germline transgenic chickens using len-tiviral vectors. EMBO Rep. 2004, 5, 728–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.S.; Koo, B.C.; Kim, D.; Nam, Y.H.; Cui, X.-S.; Kim, N.-H.; Kim, T. Generation of transgenic chickens expressing the human erythropoietin (hEPO) gene in an oviduct-specific manner: Production of transgenic chicken eggs containing human erythropoietin in egg whites. PLoS ONE 2018, 13, e0194721. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estermann, M.A.; Major, A.T.; Smith, C.A. Genetic Regulation of Avian Testis Development. Genes 2021, 12, 1459. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12091459
Estermann MA, Major AT, Smith CA. Genetic Regulation of Avian Testis Development. Genes. 2021; 12(9):1459. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12091459
Chicago/Turabian StyleEstermann, Martin Andres, Andrew Thomas Major, and Craig Allen Smith. 2021. "Genetic Regulation of Avian Testis Development" Genes 12, no. 9: 1459. https://0-doi-org.brum.beds.ac.uk/10.3390/genes12091459