Risk Stratification and Management of Arterial Hypertension and Cardiovascular Adverse Events Related to Cancer Treatments: An Oncology Network from Piedmont and Aosta Valley (North-Western Italy) Consensus Document
Abstract
:1. Introduction
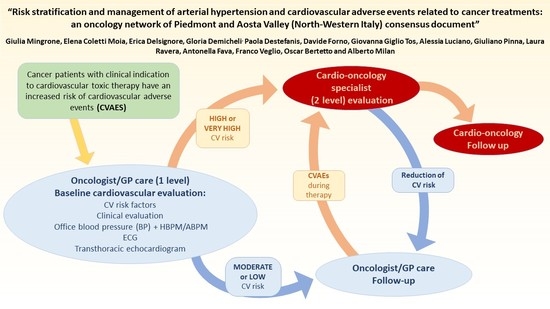
2. Cardiovascular Risk Stratification
2.1. The Workup of Cardiovascular Risk Stratification
- (1)
- Baseline evaluation (preliminary phase): identification of CV risk factors before cancer treatment initiation in order to establish the probability of developing CVAEs, to eliminate the removable risk factors and optimize CV therapy;
- (2)
- Ongoing evaluation (active phase): early diagnosis and treatment of conditions related to cardiovascular toxicity during oncologic treatment;
- (3)
- Long-term evaluation (tardive phase): to diagnose and treat tardive cardiovascular toxicity.
2.2. The Scoring System
- (1)
- High CV risk (Table 2), which includes patients:
- (a)
- With high or very high CV risk: Patients with known organ damage or high probability to develop organ damage in presence of multiple risk factors or predisposing diseases (diabetes mellitus, chronic renal insufficiency > 3 grade). These patients should be categorized at high risk to develop CV complications (such as stroke, myocardial infarction, heart failure, renal insufficiency and peripheral vasculopathy) induced by AH related to the oncologic treatment.
- (b)
- Previously treated with cardiovascular toxic therapy (high risk of iatrogenic AH): Patients previously treated with anthracyclines or other potentially cardiotoxic drugs, past chest radiotherapy, with ejection fraction reduction during previous cancer treatments, AH or CVAEs occurrence during prior therapies.
Both patients affected by known cardiopathy, vascular disease or with a SCORE risk >5% should be evaluated by a specialist in order to define the severity of the known CV disease and reveal and define the unknown organ damage (that has a high probability to be found in the presence of multiple risk factors). Similarly, patients previously treated with cardiotoxic therapy should be evaluated by a specialist in order to define the severity of organ damage. - (2)
- Moderate or low CV risk (Table 2), in which patients could be divided into:
- (a)
- With known AH: if the baseline evaluation does not reveal uncontrolled AH or subclinical organ damage, only first level diagnostic exams and routine BP monitoring should be performed in order to avoid development of AH in patients treated with VEGF inhibitors and PI. In presence of uncontrolled AH or suspected/evident subclinical organ damage at the baseline workup, second level investigations will be necessary to better define the organ damage and optimize the antihypertensive therapy.
- (b)
- Without known AH: similarly to the previous subgroup, if no AH or organ damage was revealed, only first level exams are required. On the other hand, in the presence of subclinical organ damage, a deeper specialist investigation is recommended through an ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM). This would be aimed to detect masked AH and treat accordingly. Additionally, strict BP monitoring and echocardiography are indicated to define the potential cardiac damage.
2.3. Cardiovascular Risk Stratification: Different Approach between Genders
3. Baseline Cardiovascular Evaluation
3.1. First Level Evaluation
- -
- Patient history: to identify major CV risk factors (age, gender, smoking habits, DM, AH and dyslipidemia), pre-existing CV disease or sign/symptoms suggestive of a specific unrecognized CV disease;
- -
- Evaluation of the assumed anti-hypertensive or cardiologic therapy;
- -
- Complete physical examination comprehensive of BP measurements (as recommended in ESH/ESC guidelines 2018) [13];
- -
- SCORE risk calculation;
- -
- Routine blood tests (blood cell count, creatinine, electrolytes, glycemia, uric acid, hepatic profile) along with protein electrophoresis, urine exam, microalbuminuria, TSH reflex, HbA1c, lipid and martial profile, dosage of natriuretic peptides;
- -
- Electrocardiogram;
- -
- Transthoracic echocardiogram, if possible, with global longitudinal strain (GLS) assessment [27];
- -
- ABPM and HBPM.
3.2. Second Level Evaluation
- -
- Cardio-oncological assessment with transthoracic echocardiogram (including GLS assessment);
- -
- If appropriate, according to clinical judgement: stress echocardiography, treadmill test, TSA and lower limb artery Doppler, coronary CT and cardiac MR;
- -
- Abdomen US scan in presence of altered renal function or proteinuria/microalbuminuria. If appropriate, perform a nephrological evaluation;
- -
- ABPM in presence of organ damage in patient with unknown AH in order to detect masked AH, or to optimize the antihypertensive therapy in case of uncontrolled AH;
- -
- Other specialist examinations for specific diseases identified in the first screening.
4. Diagnosis and Treatment of Arterial Hypertension
4.1. Arterial Hypertension Diagnosis
4.2. Out-of-Office BP to Reveal White Coat and Masked Hypertension
4.3. When to Begin the Antihypertensive Treatment?
4.4. Which Treatment?
- -
- Eliminate possible factors/substances which could reduce the BP control;
- -
- Identify and modify incorrect habits (obesity, scarce physical activity, alcohol assumption, high salt or low fiber diet);
- -
- Optimization of the ongoing antihypertensive therapy (synergic drug activity);
- -
- Consider secondary causes of AH.
5. Follow-Up
6. Possible Adverse Cardiovascular Effects
- -
- Proteinuria: renal protein excretion >300 mg/24 h.
- -
- Hypotension: SBP (systolic blood pressure) <90 mmHg.
- -
- Systemic: infections, volume depletions;
- -
- Iatrogenic: over-treated AH;
- -
- Dysautonomia: it is useful to verify clinostatic and orthostatic BP;
- -
- Coronary spasm/occlusion: in the presence of high risk of coronary occlusion or symptoms/signs suggestive of coronary occlusion, this suspicion must be excluded or confirmed with specific tests.
- -
- Dyspnea:
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kidoguchi, S.; Sugano, N.; Tokudome, G.; Yokoo, T.; Yano, Y.; Hatake, K.; Nishiyama, A. New Concept of Onco-Hypertension and Future Perspectives. Hypertension 2020, 77, 1–12. [Google Scholar] [CrossRef]
- Katsi, V.; Magkas, N.; Georgiopoulos, G.; Athanasiadi, E.; Virdis, A.; Masi, S.; Kliridis, P.; Hatziyanni, A.; Tsioufis, C.; Tousoulis, D. Arterial Hypertension in Patients under Antineoplastic Therapy: A Systematic Review. J. Hypertens. 2019, 37, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Puglisi, E.; Ferrari, L.; Bruno, G.; Losano, I.; Veglio, F. Arterial Hypertension and Cancer. Int. J. Cancer 2014, 134, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Tini, G.; Sarocchi, M.; Tocci, G.; Arboscello, E.; Ghigliotti, G.; Novo, G.; Brunelli, C.; Lenihan, D.; Volpe, M.; Spallarossa, P. Arterial Hypertension in Cancer: The Elephant in the Room. Int. J. Cardiol. 2019, 281, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Abi Aad, S.; Pierce, M.; Barmaimon, G.; Farhat, F.S.; Benjo, A.; Mouhayar, E. Hypertension Induced by Chemotherapeutic and Immunosuppresive Agents: A New Challenge. Crit. Rev. Oncol. Hematol. 2015, 93, 28–35. [Google Scholar] [CrossRef]
- Soultati, A.; Mountzios, G.; Avgerinou, C.; Papaxoinis, G.; Pectasides, D.; Dimopoulos, M.A.; Papadimitriou, C. Endothelial Vascular Toxicity from Chemotherapeutic Agents: Preclinical Evidence and Clinical Implications. Cancer Treat. Rev. 2012, 38, 473–483. [Google Scholar] [CrossRef]
- Herrmann, J.; Yang, E.H.; Iliescu, C.A.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.; Leesar, M.A.; Grines, C.L.; Marmagkiolis, K. Vascular Toxicities of Cancer Therapies: The Old and the New—An Evolving Avenue. Circulation 2016, 133, 1272–1289. [Google Scholar] [CrossRef]
- Plummer, C.; Michael, A.; Shaikh, G.; Stewart, M.; Buckley, L.; Miles, T.; Ograbek, A.; McCormack, T. Expert Recommendations on the Management of Hypertension in Patients with Ovarian and Cervical Cancer Receiving Bevacizumab in the UK. Br. J. Cancer 2019, 121, 109–116. [Google Scholar] [CrossRef]
- Bruno, G.; Bringhen, S.; Maffei, I.; Iannaccone, A.; Crea, T.; Ravera, A.; Astarita, A.; Vallelonga, F.; Salvini, M.; Gay, F.; et al. Cardiovascular Organ Damage and Blood Pressure Levels Predict Adverse Events in Multiple Myeloma Patients Undergoing Carfilzomib Therapy. Cancers 2019, 11, 622. [Google Scholar] [CrossRef] [Green Version]
- Bringhen, S.; De Wit, E.; Dimopoulos, M.A. New Agents in Multiple Myeloma: An Examination of Safety Profiles. Clin. Lymphoma Myeloma Leuk. 2017, 17, 391–407.e5. [Google Scholar] [CrossRef]
- Pagan, J.; Seto, T.; Pagano, M.; Cittadini, A. Role of the Ubiquitin Proteasome System in the Heart. Circ. Res. 2013, 112, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Versmissen, J.; Mirabito Colafella, K.M.; Koolen, S.L.W.; Danser, A.H.J. Vascular Cardio-Oncology: Vascular Endothelial Growth Factor Inhibitors and Hypertension. Cardiovasc. Res. 2019, 115, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for Themanagement of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Bruno, G.; Maffei, I.; Iannaccone, A.; Ravera, A.; Schiavone, D.; Veglio, F. Arterial Hypertension and Multiple Myeloma: Physiopathology and Cardiovascular Risk and ‘Practical’ Indications in Patients Receiving Carfilzomib. Curr. Hypertens. Rev. 2018, 15, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline Cardiovascular Risk Assessment in Cancer Patients Scheduled to Receive Cardiotoxic Cancer Therapies: A Position Statement and New Risk Assessment Tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society. Eur. J. Heart Fail. 2020. [Google Scholar] [CrossRef]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors. JACC CardioOncology 2019, 1, 238–251. [Google Scholar] [CrossRef]
- Bringhen, S.; Milan, A.; D’Agostino, M.; Ferri, C.; Wäsch, R.; Gay, F.; Larocca, A.; Offidani, M.; Zweegman, S.; Terpos, E.; et al. Prevention, Monitoring and Treatment of Cardiovascular Adverse Events in Myeloma Patients Receiving Carfilzomib A Consensus Paper by the European Myeloma Network and the Italian Society of Arterial Hypertension. J. Intern. Med. 2019, 286, 63–74. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Heart Score ESCardio. Available online: https:/Heartscore.Escardio.Org/2016/Quickcalculator.Aspx?Model=EuropeLow (accessed on 14 January 2021).
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.B.; Buring, J.E.; Manson, J.A.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Maffei, S.; Guiducci, L.; Cugusi, L.; Cadeddu, C.; Deidda, M.; Gallina, S.; Sciomer, S.; Gastaldelli, A.; Kaski, J.C. Women-Specific Predictors of Cardiovascular Disease Risk—New Paradigms. Int. J. Cardiol. 2019, 286, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef] [PubMed]
- Čelutkienė, J.; Pudil, R.; López-Fernández, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; Haehling, S.; et al. The Role of Cardiovascular Imaging in Cancer Patients Receiving Cardiotoxic Therapies: A Position Statement on Behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council Of. Eur. J. Heart Fail. 2020. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Cohen, J.B.; Cohen, D.L. Integrating Out-of-Office Blood Pressure in the Diagnosis and Management of Hypertension. Curr. Cardiol. Rep. 2016, 18, 112. [Google Scholar] [CrossRef] [Green Version]
- Omboni, S.; Palatini, P.; Parati, G. Standards for Ambulatory Blood Pressure Monitoring Clinical Reporting in Daily Practice: Recommendations from the Italian Society of Hypertension. Blood Press. Monit. 2015, 20, 241–244. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Cancer Therapy Evaluation Program (CTEP): Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. 2007. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 14 January 2021).
- Rosner, M.H.; Perazella, M.A. Acute Kidney Injury in Patients with Cancer. N. Engl. J. Med. 2017, 376, 1770–1781. [Google Scholar] [CrossRef]
- Siegel, D.; Martin, T.; Nooka, A.; Harvey, R.D.; Vij, R.; Niesvizky, R.; Badros, A.Z.; Jagannath, S.; McCulloch, L.; Rajangam, K.; et al. Integrated Safety Profile of Single-Agent Carfilzomib: Experience from 526 Patients Enrolled in 4 Phase II Clinical Studies. Haematologica 2013, 98, 1753–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franken, B.; van de Donk, N.W.C.J.; Cloos, J.C.; Zweegman, S.; Lokhorst, H.M. A Clinical Update on the Role of Carfilzomib in the Treatment of Relapsed or Refractory Multiple Myeloma. Ther. Adv. Hematol. 2016, 7, 330–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Drug Class | Main Molecules | Renal Damage | Cardiac Damage | Cardiovascular Toxicity | |
|---|---|---|---|---|---|
| Anti-neoplastic | Anti-angiogenic | Bevacizumab (mAb against VEGF) Sunitinib (VEGF-R inhibitor) | Yes, proteinuria | Yes, Myocardial ischemia | - Rarefaction of capillaries - Increased arterial stiffness - Endothelial dysfunction due to NO reduction |
| Proteasome inhibitor | Bortezomib Carfilzomib | Yes | Yes, heart failure, arrhythmias, myocardial ischemia | - Endothelial dysfunction - Vasoconstriction | |
| Anti-androgen | Abiraterone Enzalutamide | No | Yes | - Increase of ACTH and aldosterone levels | |
| Alkylating agent | Cisplatin Cyclophosphamide | Yes, tubular necrosis | Yes | - Endothelial and renal dysfunction -Vasoconstriction | |
| Vinca alkaloid | Vinblastine Vincristine | No | Yes | - Inhibition of endothelial cell proliferation | |
| Taxane | Paclitaxel Docetaxel | No | Yes, arrhythmias, LV dysfunction | - Inhibition of endothelial cell proliferation | |
| Anti-metabolite | Gemcitabine | Yes | Yes, myocardial ischemia | - Endothelial dysfunction - Oxidative stress | |
| HER-2 targeted | Trastuzumab | Yes, glomerulo- nephritis | Yes, LV disfunction | - Sympathetic activity - Vasoconstriction | |
| Anthracycline | Doxorubicin Daunorubicin | No | Yes, LV disfunction | - Endothelial dysfunction - Oxidative stress | |
| PI3K inhibitor | Copansilib | No | No | - Vasoconstriction | |
| Non anti- neoplastic | Hormone | Corticosteroids | No | No | - Fluid retention - Vasoconstriction |
| Immunomodulant | Ciclosporin A Tacrolimus INFα | Yes/No | No | - Sympathetic activity - Fluid retention - RAAS activity | |
| Erythropoietin | Endogenous Exogenous | No | No | - Blood viscosity - Vasoconstriction | |
| NSAID | Ibuprofen Ketoprofen | Yes | No | - Fluid retention - Prostacyclin reduction |
| Anamnestic Assessment | YES? | ||
|---|---|---|---|
| Previous myocardial infarction | □ | Very high risk | Referral to the cardiologist/ hypertension specialist |
| Coronary or other arterial revascularization procedures | □ | ||
| Acute coronary syndrome or other arterial atherosclerotic occlusions | □ | ||
| Previous stroke or transient ischemic attack | □ | ||
| Aorta aneurysm | □ | ||
| Peripheral artery disease | □ | ||
| Diabetes mellitus: with organ damage a | |||
| □ | |||
| with other major risk factor: - uncontrolled BP (grade 3) - severe dyslipidemia - smoke | □ | ||
| Severe CKD (GFR < 30 mL/min/1.73 mq) | □ | ||
| SCORE ≥ 10% | □ | ||
| Markedly elevated single risk factor: - Uncontrolled BP (grade 3) - Severe dyslipidemia (col tot >310 mg/dL) - Familiar dyslipidemia - Smoke | □ | High risk | Referral to the cardiologist/ hypertension specialist |
| Diabetes Mellitus - without organ damage a - duration ≥10 years - with an additional risk factor | |||
| □ | |||
| Moderate CKD (GFR 30–59 mL/min/1.73 mq) | □ | ||
| SCORE ≥5 and <10% | □ | ||
| TT echocardiogram: - Left ventricular hypertrophy (LVMi > 95 g/mq o ≥115 g/mq F/M); - GLS > −18% - EF < 52/54% M/F or alteration in regional/segmental contractility | □ | ||
| Uncontrolled resistant arterial hypertension (3 drugs included 1 diuretic at the full dose) | □ | ||
| Young patients with DM: - DM type 1 < 35 years; - DM type 2 < 50 years; - DM duration < 10 years; - without additional risk factors | □ | Moderate risk | Oncologist/GP care |
| SCORE ≥1% and <5% at 10 years | □ | ||
| SCORE <1% | □ | Low risk | Oncologist/GP care |
| Quality | At least 22 h of measurements and almost 1 measurement every 20 min in the day and 1 every 30 min in the night. | |
| Interpretation | Compare systolic and diastolic BP mean values during the 24 h, the day-time and the night-time with the normal ranges: | |
| SBP and/or DBP | ||
| Mean day BP value | 135 85 | |
| Mean night BP value (during sleep) | 120 70 | |
| Mean 24 h BP value | 130 80 | |
| Home BP value | 135 85 | |
| Evaluate the night BP dipping: Normal: 10% Absent < 10% Inverted Absent and inverted dipping are pathological conditions in the presence of normal sleep quality. | ||
| Night-time BP value standard deviation analysis: if > 11 mmHg, possible major impact of BP rise on organ damage. | ||
| Correlation between BP values and possible reported symptoms. | ||
| Patient History: |
| - AH duration; - HBPM; - Family history of CV events; - Habits (alcohol, smoking, recreational drug use, i.e., cocaine); - Licorice or herbal products abuse; - Lifestyle (physical inactivity, stress); - Sleep disturbances (e.g., OSA); - Drugs (antihypertensive drugs, NSAIDs, corticosteroids, collateral effects/intolerance). |
| Physical examination: |
| - Signs of secondary hypertension (hump, fat distribution, hypertrophic lower limb muscles, neck circumference, cutaneous sweating). |
| Laboratory tests: |
| - Creatinine, urea, electrolytes, glycemia, HbA1c, uric acid, TSH reflex, lipid profile, urine exam; - 24 h diuresis: quantification of fluid in-take, sodiuria, creatininuria, creatinine clearance, metanephrines dosage, urinary free cortisol; - Serum hormones: aldosterone levels, PRA. |
| Instrumental analysis: |
| - Transthoracic echocardiogram (with GLS assessment for subclinical/clinical damage); - ABPM; - Renal artery color-Doppler (or renal scintigraphy/CT/MR/arteriography based on the center’s expertise); - Polysomnography. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingrone, G.; Coletti Moia, E.; Delsignore, E.; Demicheli, G.; Destefanis, P.; Forno, D.; Giglio Tos, G.; Luciano, A.; Pinna, G.; Ravera, L.; et al. Risk Stratification and Management of Arterial Hypertension and Cardiovascular Adverse Events Related to Cancer Treatments: An Oncology Network from Piedmont and Aosta Valley (North-Western Italy) Consensus Document. Hearts 2021, 2, 61-73. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010006
Mingrone G, Coletti Moia E, Delsignore E, Demicheli G, Destefanis P, Forno D, Giglio Tos G, Luciano A, Pinna G, Ravera L, et al. Risk Stratification and Management of Arterial Hypertension and Cardiovascular Adverse Events Related to Cancer Treatments: An Oncology Network from Piedmont and Aosta Valley (North-Western Italy) Consensus Document. Hearts. 2021; 2(1):61-73. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010006
Chicago/Turabian StyleMingrone, Giulia, Elena Coletti Moia, Erica Delsignore, Gloria Demicheli, Paola Destefanis, Davide Forno, Giovanna Giglio Tos, Alessia Luciano, Giuliano Pinna, Laura Ravera, and et al. 2021. "Risk Stratification and Management of Arterial Hypertension and Cardiovascular Adverse Events Related to Cancer Treatments: An Oncology Network from Piedmont and Aosta Valley (North-Western Italy) Consensus Document" Hearts 2, no. 1: 61-73. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010006