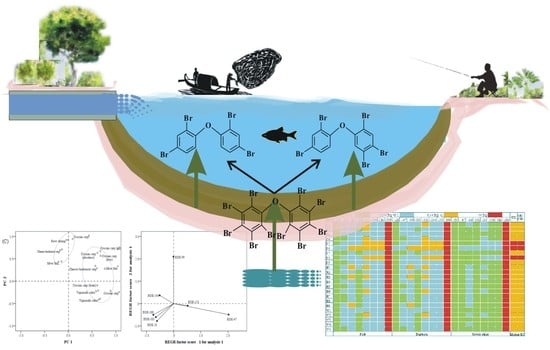
Polybrominated Diphenyl Ethers (PBDEs) in a Large, Highly Polluted Freshwater Lake, China: Occurrence, Fate, and Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Area and Sample Collection
2.3. Sample Extraction and Analyses
2.4. Quality Assurance and Quality Control
2.5. Parameter Measurement and Statistical Analysis
3. Results and Discussion
3.1. Occurrence of PBDEs in Water Collected from Chaohu Lake Basin
3.2. The Occurrence of PBDEs in Sediment Collected from Chaohu Lake Basin
3.3. Bioaccumulation of PBDEs in Biota Samples Collected from Chaohu Lake
3.4. PCA Analysis
3.5. Risk Assessment
3.5.1. Health Risk Assessment
3.5.2. Ecotoxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gerig, B.S.; Chaloner, D.T.; Janetski, D.J.; Rediske, R.R.; O’Keefe, J.P.; Moerke, A.H.; Lamberti, G.A. Congener patterns of persistent organic pollutants establish the extent of contaminant biotransport by Pacific salmon in the Great Lakes. Environ. Sci. Technol. 2015, 50, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Ma, Y.J.; Wang, J.; Tian, M.; Luo, X.; Chen, D.; Mai, B. Measurement and human exposure assessment of brominated flame retardants in household products from south China. J. Hazard. Mater. 2010, 176, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Betts, K. Does a key PBDE break down in the environment? Environ. Sci. Technol. 2008, 42, 6781. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lu, Y.; Wang, T.; Kannan, K.; Gosens, J.; Xu, L.; Li, Q.; Wang, L.; Liu, S. A review of human exposure to Polybrominated diphenyl ethers (PBDEs) in China. Int. J. Hyg. Environ. Heal. 2013, 216, 607–623. [Google Scholar] [CrossRef] [PubMed]
- La Guardia, M.J.; Hale, R.; Harvey, E. Evidence of debromination of decabromodiphenyl ether (BDE-209) in biota from a wastewater receiving stream. Environ. Sci. Technol. 2007, 41, 6663–6670. [Google Scholar] [CrossRef] [PubMed]
- Lagalante, A.F.; Shedden, C.S.; Greenbacker, P.W. Levels of Polybrominated diphenyl ethers (PBDEs) in dust from personal automobiles in conjunction with studies on the photochemical degradation of decabromodiphenyl ether (BDE-209). Environ. Int. 2011, 37, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, E.; Covaci, A.; Jaspers, V.L.B.; Dauwe, T.; Voorspoels, S.; Eens, M.; Pinxten, R. Accumulation, tissue-specific distribution and debromination of decabromodiphenyl ether (BDE 209) in european starlings (Sturnus vulgaris). Environ. Pollut. 2007, 148, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Wen, Z.H.; Duan, Y.; Lu, Z.; Meng, X.; Zhang, W. Characterizing distribution, sources, and potential health risk of Polybrominated diphenyl ethers (PBDEs) in office environment. Environ. Pollut. 2015, 198, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, Q.; Liu, X.; Wang, J. Occurrence, distribution and risk assessment of Polychlorinated biphenyls and Polybrominated diphenyl ethers in nine water sources. Ecotoxicol. Environ. Saf. 2015, 115, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shang, H.; Li, H.; Wang, Y.; Li, Y.; Zhang, H.; Zhang, Q.; Liang, Y.; Jiang, G. PBDEs, PCBs and PCDD/FS in the sediments from seven major river basins in China: Occurrence, congener profile and spatial tendency. Chemosphere 2016, 144, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, G.; Lam, M.H.W.; Liu, H.; Da, C. Occurrence and levels of Polybrominated diphenyl ethers in surface sediments from the Yellow River estuary, China. Environ. Pollut. 2016, 212, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, H.; Chen, J.; Qiao, X.; Xie, Q.; Zhang, Y. Polybrominated diphenyl ethers in soils of the modern Yellow River Delta, China: Occurrence, distribution and inventory. Chemosphere 2012, 88, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.H.; Luo, X.J.; Chen, S.J.; Yu, M.; Mai, B.; Zeng, E. Polybrominated diphenyl ethers in biota and sediments of the Pearl River estuary, south China. Environ. Toxicol. Chem. 2007, 26, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Lavandier, R.; Arêas, J.; Quinete, N.; de Moura, J.F.; Taniguchi, S.; Montone, R.; Siciliano, S.; Moreira, I. PCB and PBDE levels in a highly threatened dolphin species from the southeastern Brazilian coast. Environ. Pollut. 2016, 208, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lee, S.; Jeong, Y.; Yu, J.P.; Baek, W.K.; Kannan, K.; Moon, H.B. Species-specific accumulation of Polybrominated diphenyl ethers (PBDEs) and other emerging flame retardants in several species of birds from Korea. Environ. Pollut. 2016, 219, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, C.; Butt, C.M.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of Polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ. Int. 2016, 88, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Bellés, M.; Domingo, J.L. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 2015, 89, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Liu, L.Y.; Zhang, K.; Liang, B.; Li, G.L.; Chen, T.H. Halogenated organic contaminants (HOCs) in sediment from a highly eutrophicated lake, China: Occurrence, distribution and mass inventories. Chemosphere 2012, 89, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Martí-Cid, R.; Bocio, A.; Llobet, J.M.; Domingo, J.L. Intake of chemical contaminants through fish and seafood consumption by children of Catalonia, Spain: Health risks. Food Chem. Toxicol. 2007, 45, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, B.; Huo, S.; Deng, L.; Pan, H.; Xia, X.; Zhang, J.; Ren, Y.; Liu, H. Polybrominated diphenyl ethers occurrence in major inflowing rivers of Lake Chaohu (China): Characteristics, potential sources and inputs to lake. Chemosphere 2013, 93, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, B.; Huo, S.; Sun, W.; Pan, H.; Zhang, J.; Ren, Y.; Liu, H. Characterization, treatment and releases of PBDEs and PAHs in a typical municipal sewage treatment plant situated beside an urban river, east China. J. Environ. Sci. 2013, 25, 1281–1290. [Google Scholar] [CrossRef]
- North, K.D. Tracking Polybrominated diphenyl ether releases in a wastewater treatment plant effluent, Palo Alto, California. Environ. Sci. Technol. 2004, 38, 4484–4488. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.F.; Wang, J.Z.; Ni, H.G.; Zeng, E.Y. Riverine inputs of Polybrominated diphenyl ethers from the Pearl River Delta (China) to the coastal ocean. Environ. Sci. Technol. 2007, 41, 6007–6013. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yao, H.; Wang, H.; Li, H.; Lu, S.; Zhang, X.; Xiang, X. Polybrominated diphenyl ethers (PBDEs) in water, surface sediment, and suspended particulate matter from the Yellow River, China: Levels, spatial and seasonal distribution, and source contribution. Mar. Pollut. Bull. 2018, 129, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yun, X.; Liu, M.; Yang, Y.; Zhang, M.; Wang, J. Distribution, potential source and ecotoxicological risk of Polychlorinated biphenyls and Polybrominated diphenyl ethers in the surface water of the three gorges dam region of the Yangtze River, China. Ecotoxicology 2014, 23, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Chen, X.R.; Zhao, Y.X.; Li, Y.; Qin, X.; Qin, Z. Changes of Polybrominated diphenyl ether concentrations in ducks with background exposure level and time. Chemosphere 2015, 118, 253–260. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Qin, N.; Kong, X.; Liu, W.; He, Q.; Ouyang, H.; Wang, Q.; Yang, B.; Yang, C.; Jiang, Y.; et al. Polybrominated diphenyl ethers (PBDEs) in the surface sediments and suspended particulate matter (SPM) from Lake Chaohu, a large shallow Chinese lake. Sci. Total Environ. 2013, 463–464, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, J.A.; Ahn, M.Y.; Leng, J.; Filley, T.R.; Nies, L. Reductive debromination of Polybrominated diphenyl ethers in anaerobic sediment and a biomimetic system. Environ. Sci. Technol. 2008, 42, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, G.; Peifang, W.; Wu, H.; Qi, P.; Liang, Y. Assessment of environmental pollution of Taihu Lake by combining active biomonitoring and integrated biomarker response. Environ. Sci. Technol. 2011, 45, 3746–3752. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Zhang, K.; Liang, B.; Zeng, E.Y. Occurrence, source apportionment and toxicity assessment of polycyclic aromatic hydrocarbons in surface sediments of Chaohu, one of the most polluted lakes in China. J. Environ. Monit. 2011, 13, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, J.; Chang, H.; Liao, H.; Zhao, X.; Mai, B.; Xing, B. Polybrominated diphenyl ethers and decabromodiphenylethane in sediments from twelve lakes in China. Environ. Pollut. 2012, 162, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Z.; Zeng, E.Y.; Yu, L.P.; Mai, B.; Luo, X.; Ran, Y. Persistent halogenated hydrocarbons in consumer fish of China: Regional and global implications for human exposure. Environ. Sci. Technol. 2007, 41, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Wu, J.P.; Luo, X.J.; Sun, Y.; Zheng, X.; Zhang, Q.; Zou, F.; Mai, B. Dechlorane plus flame retardant in kingfishers (Alcedo atthis) from an electronic waste recycling site and a reference site, south China: Influence of residue levels on the isomeric composition. Environ. Pollut. 2013, 174, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Stahl, L.L.; Snyder, B.D.; Olsen, A.R.; Walters, L.S. A national probabilistic study of Polybrominated diphenyl ethers in fish from US lakes and reservoirs. Environ. Monit. Assess. 2013, 185, 10351–10364. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.; Gewurtz, S.B.; Drouillard, K.G.; Kolic, T.; MacPherson, K.; Reiner, E.J.; Bhavsar, S.P. Polybrominated diphenyl ethers (PBDEs) in Great Lakes fish: Levels, patterns, trends and implications for human exposure. Sci. Total Environ. 2017, 576, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, R.; Hallikainen, A.; Rantakokko, P.; Ruokojärvi, P.; Vuorinen, P.J.; Mannio, J.; Kiviranta, H. Levels and congener profiles of PBDEs in edible Baltic, freshwater, and farmed fish in Finland. Environ. Sci. Technol. 2015, 49, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.C.; Dai, J.Y.; Xu, Z.C.; Luo, X.; Cao, H.; Wang, J.; Mai, B.; Xu, M. Bioaccumulation behavior of Polybrominated diphenyl ethers (PBDEs) in the freshwater food chain of Baiyangdian Lake, north China. Environ. Int. 2010, 36, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lam, J.C.W.; Man, Y.C.; Lai, N.L.; Kwok, K.Y.; Guo, Y.; Lam, P.K.; Zhou, B. Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat. Toxicol. 2015, 158, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.G.; de Laat, R.; Tagliaferri, S.; Pellacani, C. A mechanistic view of Polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014, 230, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, G.; Liu, J.; Yan, Z.; Zhang, Z. Bioaccumulation, distribution and metabolism of BDE-153 in the freshwater fish Carassius auratus after dietary exposure. Ecotoxicol. Environ. Saf. 2014, 108, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ondarza, P.M.; Gonzalez, M.; Fillmann, G.; Miglioranza, K.S.B. PBDEs, PCBs and organochlorine pesticides distribution in edible fish from Negro River basin, Argentinean Patagonia. Chemosphere 2014, 94, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. Polybrominated diphenyl ethers in food and human dietary exposure: A review of the recent scientific literature. Food Chem. Toxicol. 2012, 50, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Environment Canada 2013. Federal Environmental Quality Guidelines (FEQGs) for Polybrominated Diphenyl Ethers (PBDEs). Available online: http://www.ec.gc.ca/ese-ees/05DF7A37-60FF-403F-BB37-0CC697DBD9A3/FEQG PBDE EN.pdf (accessed on 17 July 2018).
- Yu, Y.; Wang, X.; Yang, D.; Lei, B.; Zhang, X.; Zhang, X. Evaluation of human health risks posed by carcinogenic and non-carcinogenic multiple contaminants associated with consumption of fish from Taihu Lake, China. Food Chem. Toxicol. 2014, 69, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Martellini, T.; Diletti, G.; Scortichini, G.; Lolini, M.; Lanciotti, E.; Katsoyiannis, A.; Cincinelli, A. Occurrence of polybrominated diphenyl ethers (PBDEs) in foodstuffs in Italy and implications for human exposure. Food Chem. Toxicol. 2016, 89, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, G.; An, T.; Zhang, C.; Wei, C. Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang River, South China. Environ. Pollut. 2016, 219, 596–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhaus, T.; Karlsson, M. Screening level mixture risk assessment of pharmaceuticals in STP effluents. Water Res. 2014, 49, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lu, G.; Zhang, F.; Nkoom, M.; Yan, Z.; Wu, D. Polybrominated Diphenyl Ethers (PBDEs) in a Large, Highly Polluted Freshwater Lake, China: Occurrence, Fate, and Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 1529. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15071529
Liu J, Lu G, Zhang F, Nkoom M, Yan Z, Wu D. Polybrominated Diphenyl Ethers (PBDEs) in a Large, Highly Polluted Freshwater Lake, China: Occurrence, Fate, and Risk Assessment. International Journal of Environmental Research and Public Health. 2018; 15(7):1529. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15071529
Chicago/Turabian StyleLiu, Jianchao, Guanghua Lu, Fuhai Zhang, Matthew Nkoom, Zhenhua Yan, and Donghai Wu. 2018. "Polybrominated Diphenyl Ethers (PBDEs) in a Large, Highly Polluted Freshwater Lake, China: Occurrence, Fate, and Risk Assessment" International Journal of Environmental Research and Public Health 15, no. 7: 1529. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph15071529