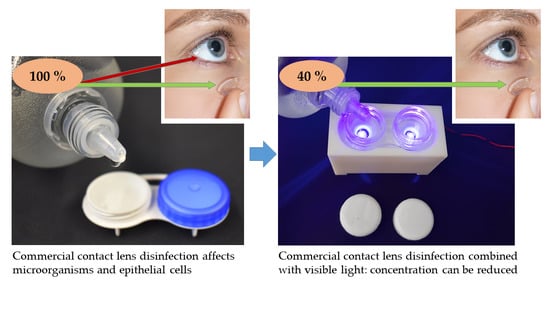
Enhancement of Contact Lens Disinfection by Combining Disinfectant with Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Contact Lens Disinfection Solutions
2.2. Irradiation Setup
2.3. Disk Diffusion Assay
2.4. Determination of Bacterial Reduction with Nutrient Pads
2.5. Determination of Bacterial Reduction with Agar Plates
2.6. Determination of Bacterial Reduction via Regrowth Behavior
3. Results
3.1. Disk Diffusion Assay
3.2. Determination of Bacterial Reduction with Nutrient Pads
3.3. Determination of Bacterial Reduction with Agar Plates
3.4. Loewe Additivity
3.5. Effectiveness Dependency of Multipurpose Solution ReNu Multiplus on Bacterial Concentration
3.6. Determination of Bacterial Reduction via Regrowth Behavior
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Barr, J.T. 2004 Annual Report; Contact Lens Spectrum: Ambler, PA, USA, 2005; pp. 26–32. [Google Scholar]
- Cavanagh, H.D.; Robertson, D.M.; Petroll, W.M.; Jester, J.V. Castroviejo Lecture 2009: 40 Years in Search of the Perfect Contact Lens. Cornea 2010, 29, 1075–1085. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.W. A cross-sectional analysis of U.S. contact lens user demographics. Optom. Vis. Sci. 2012, 89, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Fagan, X.J.; Jhanji, V.; Constantinou, M.; Islam, F.M.A.; Taylor, H.R.; Vajpayee, R.B. First contact diagnosis and management of contact lens-related complications. Int. Ophthalmol. 2012, 32, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.; Hume, E.B.H.; Vijay, A.K.; Sankaridurg, P.; Kumar, N.; Willcox, M.D. In Vivo Performance of Melimine as an Antimicrobial Coating for Contact Lenses in Models of CLARE and CLPU. Investig. Opthalmol. Vis. Sci. 2010, 51, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, F.; Ramachandran, L.; Sweeney, D.F.; Rao, G.; Holden, B.A. Altered Conjunctival Response after Contact Lens–Related Corneal Inflammation. Cornea 2003, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Szczotka-Flynn, L.B.; Pearlman, E.; Ghannoum, M. Microbial Contamination of Contact Lenses, Lens Care Solutions, and Their Accessories: A Literature Review. Eye Contact Lens 2010, 36, 116–129. [Google Scholar] [CrossRef]
- Stapleton, F.; Keay, L.; Edwards, K.; Naduvilath, T.; Dart, J.K.; Brian, G.; Holden, B.A. The Incidence of Contact Lens–Related Microbial Keratitis in Australia. Ophthalmology 2008, 115, 1655–1662. [Google Scholar] [CrossRef]
- Seal, D.; Kirkness, C.; Bennett, H.; Peterson, M. Population-Based cohort study of microbial keratitis in Scotland: Incidence and features. Contact Lens Anterior Eye 1999, 22, 49–57. [Google Scholar] [CrossRef]
- Lam, D.S.C.; Houang, E.; Fan, D.S.P.; Lyon, D.; Seal, D.; Wong, E. Incidence and risk factors for microbial keratitis in Hong Kong: Comparison with Europe and North America. Eye 2002, 16, 608–618. [Google Scholar] [CrossRef]
- Keay, L.; Stapleton, F.; Schein, O. Epidemiology of Contact Lens-Related Inflammation and Microbial Keratitis: A 20-year Perspective. Eye Contact Lens 2007, 33, 346–353. [Google Scholar] [CrossRef]
- Carnt, N.; Samarawickrama, C.; White, A.; Stapleton, F. The diagnosis and management of contact lens-related microbial keratitis. Clin. Exp. Optom. 2017, 100, 482–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadinia, M.; Rahmani, S.; Eslami, G.; Ghassemi-Broumand, M.; Amiri, M.A.; Aghaie, G.; Tabatabaee, S.M.; Taheri, S.; Behgozin, A. Contact lens disinfecting solutions antibacterial efficacy: Comparison between clinical isolates and the standard ISO ATCC strains of Pseudomonas aeruginosa and Staphylococcus aureus. Eye 2011, 26, 327–330. [Google Scholar] [PubMed] [Green Version]
- Boost, M.; Lai, S.; Ma, C.; Cho, P. Do multipurpose contact lens disinfecting solutions work effectively against non-FDA/ISO recommended strains of bacteria and fungi? Ophthalmic Physiol. Opt. 2010, 30, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, C.; Fleiszig, S.M.J. Resistance of Pseudomonas aeruginosa Isolates to Hydrogel Contact Lens Disinfection Correlates with Cytotoxic Activity. J. Clin. Microbiol. 2001, 39, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.M.; Petroll, W.M.; Jester, J.V.; Cavanagh, H.D. The Role of Contact Lens Type, Oxygen Transmission, and Care-Related Solutions in Mediating Epithelial Homeostasis and Pseudomonas Binding to Corneal Cells: An Overview. Eye Contact Lens 2007, 33, 394–398. [Google Scholar] [CrossRef]
- Posch, L.C.; Zhu, M.; Robertson, D.M. Multipurpose Care Solution–Induced Corneal Surface Disruption and Pseudomonas aeruginosa Internalization in the Rabbit Corneal Epithelium. Investig. Opthalmol. Vis. Sci. 2014, 55, 4229–4237. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.A.; Maltseva, I.A.; Rogers, V.A.; Ni, J.; Khong, K.T.; Derringer, C.B.; George, M.D.; Luk, A.S. Consequences of Preservative Uptake and Release by Contact Lenses. Eye Contact Lens 2018, 44, S247–S255. [Google Scholar] [CrossRef]
- Tchao, R.; McCanna, D.J.; Miller, M.J. Comparison of contact lens multipurpose solutions by in vitro sodium fluorescein permeability assay. Eye Contact Lens 2002, 28, 151–156. [Google Scholar]
- Imayasu, M.; Shiraishi, A.; Ohashi, Y.; Shimada, S.; Cavanagh, H.D. Effects of Multipurpose Solutions on Corneal Epithelial Tight Junctions. Eye Contact Lens 2008, 34, 50–55. [Google Scholar] [CrossRef]
- Erdinest, N.; Ovadia, H.; Solomon, A. Cytotoxic and Inflammatory Effects of Contact Lens Multipurpose Solutions on Human Corneal Epithelial Cells. Eur. J. Inflamm. 2013, 11, 145–160. [Google Scholar] [CrossRef]
- Dutot, M.; Paillet, H.; Chaumeil, C.; Warnet, J.-M.; Rat, P. Severe ocular infections with contact lens: Role of multipurpose solutions. Eye 2008, 23, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutot, M.; Warnet, J.-M.; Baudouin, C.; Rat, P. Cytotoxicity of contact lens multipurpose solutions: Role of oxidative stress, mitochondrial activity and P2X7 cell death receptor activation. Eur. J. Pharm. Sci. 2008, 33, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Tanti, N.C.; Jones, L.; Gorbet, M.B. Impact of multipurpose solutions released from contact lenses on corneal cells. Optom. Vis. Sci. 2011, 88, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Reveneau, E.; Pauloin, T.; Fagon, R.; Tanter, C.; Warnet, J.-M.; Rat, P. Multipurpose Solutions and Contact Lens: Modulation of Cytotoxicity and Apoptosis on the Ocular Surface. Cornea 2010, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.; Cho, P.; Boost, M.V. Cytotoxicity and effects on metabolism of contact lens care solutions on human corneal epithelium cells. Clin. Exp. Optom. 2012, 95, 198–206. [Google Scholar] [CrossRef]
- Ahmed, I.; Fang, Y.; Lu, M.; Yan, Q.; El-Hussein, A.; Hamblin, M.R.; Dai, T.; Kamel, A.E.-H.M. Recent Patents on Light-Based Anti-Infective Approaches. Recent Pat. Anti-Infect. Drug Discov. 2018, 13, 70–88. [Google Scholar] [CrossRef]
- Cabral, J.; Rodrigues, A.G. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics 2019, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Update 2012, 15, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Lubart, R.; Lipovski, A.; Nitzan, Y.; Friedmann, H. A Possible Mechanism for the Bactericidal Eeffect of Visible Light. Laser Ther. 2011, 20, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of Bacterial Pathogens following Exposure to Light from a 405-Nanometer Light-Emitting Diode Array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef] [Green Version]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths-A review on existing data. FEMS Microbiol. Lett. 2016, 364. [Google Scholar] [CrossRef] [PubMed]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; MacLean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Tomb, R.M.; MacLean, M.; Coia, J.E.; Graham, E.; McDonald, M.; Atreya, C.D.; MacGregor, S.J.; Anderson, J.G. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light against Feline Calicivirus as a Model for Norovirus Decontamination. Food Environ. Virol. 2016, 9, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Tomb, R.M.; Maclean, M.; Herron, P.R.; Hoskisson, P.A.; MacGregor, S.J.; Anderson, J.G. Inactivation of Streptomyces phage ɸC31 by 405 nm light: Requirement for exogenous photosensitizers? Bacteriophage 2014, 4, e32129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenes, K.; Stangl, F.; Gross, A.; Hessling, M. Improved contact lens disinfection by exposure to violet radiation. Technol. Health Care 2016, 24, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hoenes, K.; Wenzel, U.; Hessling, M. Realisation and assessment of a low-cost LED device for contact lens disinfection by visible violet light. Biomed. Tech. 2019, 65, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Rapacka-Zdonczyk, A.; Mutters, N.T.; Grinholc, M. Antimicrobials Are a Photodynamic Inactivation Adjuvant for the Eradication of Extensively Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Lamberti, M.J.; Vittar, N.B.R.; Da Silva, F.; Ferreira, V.; Rivarola, V.A. Synergistic enhancement of antitumor effect of β-Lapachone by photodynamic induction of quinone oxidoreductase (NQO1). Phytomedicine 2013, 20, 1007–1012. [Google Scholar] [CrossRef]
- Fila, G.; Kawiak, A.; Grinholc, M. Blue light treatment ofPseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence 2016, 8, 938–958. [Google Scholar] [CrossRef] [Green Version]
- Moorhead, S.; MacLean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Synergistic efficacy of 405 nm light and chlorinated disinfectants for the enhanced decontamination of Clostridium difficile spores. Anaerobe 2016, 37, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Akram, F.E.; El-Tayeb, T.; Abou-Aisha, K.; El-Azizi, M. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Ann. Clin. Microbiol. Antimicrob. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, O.; Moreinos, D.; Steinberg, D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J. Antimicrob. Chemother. 2006, 57, 872–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, P.; Mohammad, H.; Hui, J.; Leanse, L.G.; Li, J.; Liang, L.; Dai, T.; Seleem, M.N.; Cheng, J.-X. Photolysis of Staphyloxanthin in Methicillin-Resistant Staphylococcus aureus Potentiates Killing by Reactive Oxygen Species. Adv. Sci. 2019, 6, 1900030. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial Photodynamic Inactivation Mediated by Methylene Blue and Red Light Is Enhanced by Synergistic Effect of Potassium Iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, L.M.; Soares, C.P.; Fontana, C.R. Synergistic effect of photodynamic therapy and cisplatin: A novel approach for cervical cancer. J. Photochem. Photobiol. B Boil. 2014, 140, 365–373. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.K.; Chae, H.S. Synergistic in vitro photodynamic antimicrobial activity of methylene blue and chitosan against Helicobacter pylori 26695. Photodiagn. Photodyn. Ther. 2014, 11, 526–532. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Donnelly, R.F.; Elborn, J.; Magee, N.D.; Tunney, M.M. Photodynamic Antimicrobial Chemotherapy (PACT) in combination with antibiotics for treatment of Burkholderia cepacia complex infection. J. Photochem. Photobiol. B Boil. 2012, 106, 95–100. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewe, S.T.; Muischnek, H. Über Kombinationswirkungen. Naunyn-Schmiedebergs Arch. Pharmacol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Lin, L.; Kim, J.; Chen, H.; Kowalski, R.; Nizet, V. Component Analysis of Multipurpose Contact Lens Solutions To Enhance Activity against Pseudomonas aeruginosa and Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 4259–4263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; E Bond, A.; Pan, N.; Coleman, M.; Tang, Y.; Sun, Y.-P.; Yang, L. Synergistic photoactivated antimicrobial effects of carbon dots combined with dye photosensitizers. Int. J. Nanomed. 2018, 13, 8025–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, D.T.; Bui, M.-T.; Memon, P.; Cavanagh, H.D. Microbial Keratitis at an Urban Public Hospital: A 10-Year Update. J. Clin. Exp. Ophthalmol. 2015, 6, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, C.-F.; Tseng, C.-H.; Hu, F.-R.; Wang, I.-J.; Chen, W.-L.; Hou, Y.-C. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am. J. Ophthalmol. 2004, 137, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D. Pseudomonas aeruginosa Infection and Inflammation during Contact Lens Wear: A Review. Optom. Vis. Sci. 2007, 84, 273–278. [Google Scholar] [CrossRef]
- Stapleton, F.; Keay, L.; Sanfilippo, P.G.; Katiyar, S.; Edwards, K.P.; Naduvilath, T. Relationship between Climate, Disease Severity, and Causative Organism for Contact Lens–Associated Microbial Keratitis in Australia. Am. J. Ophthalmol. 2007, 144, 690–698. [Google Scholar] [CrossRef]
- McLaughlin, -B.L.; Stapleton, F.; Matheson, M.; Dart, J.K.G. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol. 1998, 84, 827–838. [Google Scholar] [CrossRef]
- ISO-International Organization of Standards. ISO 14729:2001/AMD 1:2010 Ophthalmic Optics-Contact Lens Care Products-Microbiological Requirements and Test Methods for Products and Regimens for Hygienic Management of Contact Lenses. Available online: https://www.beuth.de/de/erweiterte-suche/272754!search?alx.searchType=complex&searchAreaId=1&query=DIN+EN+ISO+14729+&facets%5B276612%5D=&hitsPerPage=10 (accessed on 10 July 2020).
- Hoenes, K.; Wenzel, U.; Spellerberg, B.; Hessling, M. Photoinactivation Sensitivity of Staphylococcus carnosus to Visible-light Irradiation as a Function of Wavelength. Photochem. Photobiol. 2019, 96, 156–169. [Google Scholar] [CrossRef]
- McCanna, D.J.; Harrington, K.L.; Driot, J.-Y.; Ward, K.W.; Tchao, R. Use of a Human Corneal Epithelial Cell Line for Screening the Safety of Contact Lens Care Solutions In Vitro. Eye Contact Lens 2008, 34, 6–12. [Google Scholar] [CrossRef]
- Schmid, J.; Hoenes, K.; Vatter, P.; Hessling, M. Antimicrobial Effect of Visible Light—Photoinactivation of Legionella rubrilucens by Irradiation at 450, 470, and 620 nm. Antibiotics 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plavskii, V.; Mikulich, A.; Tretyakova, A.; Leusenka, I.; Plavskaya, L.; Kazyuchits, O.; Dobysh, I.; Krasnenkova, T. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B Boil. 2018, 183, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 2016, 7.5, 536–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashef, N.; Akbarizare, M.; Kamrava, S.K. Effect of sub-lethal photodynamic inactivation on the antibiotic susceptibility and biofilm formation of clinical Staphylococcus aureus isolates. Photodiagn. Photodyn. Ther. 2013, 10, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Kilvington, S.; Shovlin, J.; Nikolic, M. Identification and susceptibility to multipurpose disinfectant solutions of bacteria isolated from contact lens storage cases of patients with corneal infiltrative events. Contact Lens Anterior Eye 2013, 36, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Angarano, V.; Akkermans, S.; Smet, C.; Chieffi, A.; Van Impe, J.F.M. The potential of violet, blue, green and red light for the inactivation of P. fluorescens as planktonic cells, individual cells on a surface and biofilms. Food Bioprod. Process. 2020, in press. [Google Scholar] [CrossRef]
- Gupta, S.; MacLean, M.; Anderson, J.; MacGregor, S.J.; Meek, R.M.D.; Grant, M. Inactivation of micro-organisms isolated from infected lower limb arthroplasties using high-intensity narrow-spectrum (HINS) light. Bone Jt. J. 2015, 97, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, R.G.; Rose, J.B.; Hashsham, S.A.; Gerba, C.P.; Haas, C.N. Criteria for Selection of Surrogates Used To Study the Fate and Control of Pathogens in the Environment. Appl. Environ. Microbiol. 2012, 78, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.T.; Wooten, D.J.; Paudel, B.B.; Bauer, J.; Hardeman, K.N.; Westover, D.; Lovly, C.M.; Harris, L.A.; Tyson, D.R.; Quaranta, V. Quantifying Drug Combination Synergy along Potency and Efficacy Axes. Cell Syst. 2019, 8, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Gorbet, M.B.; Tanti, N.; Crockett, B.; Mansour, L.; Jones, L. Effect of contact lens material on cytotoxicity potential of multipurpose solutions using human corneal epithelial cells. Mol. Vis. 2011, 17, 3458–3467. [Google Scholar]
- Lehmann, D.M.; Richardson, M.E. Impact of assay selection and study design on the outcome of cytotoxicity testing of medical devices: The case of multi-purpose vision care solutions. Toxicol. Vitr. 2010, 24, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Heiler, D.; Norton, S. Report on Testing From an Investigation of Fusarium Keratitis in Contact Lens Wearers. Eye Contact Lens 2006, 32, 256–261. [Google Scholar] [CrossRef] [PubMed]






| Combination | a | b | A | B | CI | |
|---|---|---|---|---|---|---|
| Log | Dose in J/cm2 | % | Dose in J/cm2 | % | ||
| 10 mW/cm2 | −0.82 | 140 | 5 | 137.7 | 24.8 | 1.2186 |
| −2.19 | 140 | 20 | 230.6 | 47.5 | 1.0278 | |
| −3.26 | 140 | 30 | 303.1 | 65.3 | 0.9215 | |
| −3.86 | 140 | 40 | 344.0 | 75.3 | 0.9382 | |
| 20 mW/cm2 | −1.12 | 140 | 5 | 169.3 | 29.5 | 0.9963 |
| −2.76 | 140 | 20 | 327.8 | 60.1 | 0.7600 | |
| −4.09 | 140 | 30 | 457.0 | 84.9 | 0.6595 | |
| −4.49 | 140 | 40 | 496.5 | 92.5 | 0.7142 | |
| 40 mW/cm2 | −0.76 | 140 | 5 | 226.1 | 28.4 | 0.7955 |
| −0.73 | 140 | 20 | 223.1 | 27.8 | 1.3460 | |
| −1.29 | 140 | 30 | 282.5 | 37.0 | 1.2841 | |
| −2.01 | 140 | 40 | 359.1 | 51.2 | 1.1708 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoenes, K.; Spellerberg, B.; Hessling, M. Enhancement of Contact Lens Disinfection by Combining Disinfectant with Visible Light Irradiation. Int. J. Environ. Res. Public Health 2020, 17, 6422. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17176422
Hoenes K, Spellerberg B, Hessling M. Enhancement of Contact Lens Disinfection by Combining Disinfectant with Visible Light Irradiation. International Journal of Environmental Research and Public Health. 2020; 17(17):6422. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17176422
Chicago/Turabian StyleHoenes, Katharina, Barbara Spellerberg, and Martin Hessling. 2020. "Enhancement of Contact Lens Disinfection by Combining Disinfectant with Visible Light Irradiation" International Journal of Environmental Research and Public Health 17, no. 17: 6422. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17176422