A Bibliometric Analysis of the Literature on Irisin from 2012–2021
Abstract
:1. Introduction
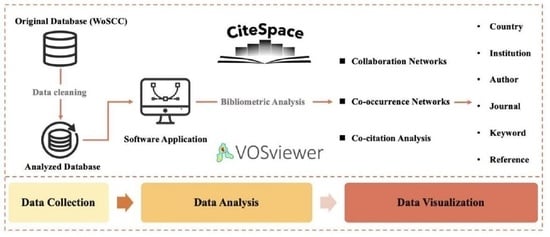
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Analysis Methods
3. Results
3.1. Publication Outputs
3.2. Country Analysis
3.3. Institution Analysis
3.4. Co-Authorship Analysis
3.5. Journal Analysis
3.6. Analysis of Keywords Co-Occurrence
3.7. Analysis of Reference Co-Citation
4. Discussion
4.1. General Information
4.2. Research Hotspots
4.2.1. Irisin and Insulin Resistance
4.2.2. Irisin and Inflammation
4.2.3. Circulating Irisin Level in Serum
4.3. Research Frontiers
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panati, K.; Suneetha, Y.; Narala, V.R. Irisin/FNDC5—An updated review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 689–697. [Google Scholar]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e1717. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, F.; Zamani, S.; Barreiro, C.; Jami, M.S. Irisin injection mimics exercise effects on the brain proteome. Eur. J. Neurosci. 2021, 54, 7422–7441. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Ibekwe-SanJuan, F.; Hou, J.H. The Structure and Dynamics of Cocitation Clusters: A Multiple-Perspective Cocitation Analysis. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 1386–1409. [Google Scholar] [CrossRef] [Green Version]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.M. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Qi, B.; Jin, S.; Qian, H.; Zou, Y. Bibliometric Analysis of Chronic Traumatic Encephalopathy Research from 1999 to 2019. Int. J. Environ. Res. Public Health 2020, 17, 5411. [Google Scholar] [CrossRef]
- Liao, H.C.; Tang, M.; Luo, L.; Li, C.Y.; Chiclana, F.; Zeng, X.J. A Bibliometric Analysis and Visualization of Medical Big Data Research. Sustainability 2018, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Hu, Z.; Liu, S.; Tseng, H. Emerging trends in regenerative medicine: A scientometric analysis in CiteSpace. Expert Opin. Biol. Ther. 2012, 12, 593–608. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernandez-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Liu, J.J.; Wong, M.D.S.; Toy, W.C.; Tan, C.S.H.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Muggeo, M. Prevalence of insulin resistance in metabolic disorders: The Bruneck Study. Diabetes 1998, 47, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Lin, M.; Liu, C.; Xiao, F.; Liu, Y.; Huang, P.; Zeng, X.; Yan, B.; Liu, S.; Li, X.; et al. Elevated circulating irisin is associated with lower risk of insulin resistance: Association and path analyses of obese Chinese adults. BMC Endocr. Disord. 2016, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Belviranli, M.; Okudan, N.; Çelik, F. Association of Circulating Irisin with Insulin Resistance and Oxidative Stress in Obese Women. Horm. Metab. Res. 2016, 48, 653–657. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, M.K.; Bae, K.H.; Seo, H.A.; Jeong, J.Y.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Park, K.G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Zügel, M.; Sun, Z.; Steinacker, J.M.; Schumann, U. Association between circulating irisin and insulin resistance in non-diabetic adults: A meta-analysis. Metabolism 2016, 65, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6490–6497. [Google Scholar]
- Zheng, S.; Chen, N.; Kang, X.; Hu, Y.; Shi, S. Irisin alleviates FFA induced β-cell insulin resistance and inflammatory response through activating PI3K/AKT/FOXO1 signaling pathway. Endocrine 2022, 75, 740–751. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, P.; Chen, S.; Sun, Q.; Zeng, Q.C.; Chen, J.Y.; Liu, Y.X.; Cao, X.H.; Ren, M.; Wang, J.K. The association of new inflammatory markers with type 2 diabetes mellitus and macrovascular complications: A preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1567–1572. [Google Scholar] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Bilski, J.; Pochec, E.; Brzozowski, T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J. Physiol. Pharmacol. 2017, 68, 243–251. [Google Scholar] [PubMed]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered metabolism in mice lacking irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, T.; Leung, P.S. Irisin Ameliorates Glucolipotoxicity-Associated β-Cell Dysfunction and Apoptosis via AMPK Signaling and Anti-Inflammatory Actions. Cell Physiol. Biochem. 2018, 51, 924–937. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Yuan, C.; Li, R.; Ma, Y.; Tang, X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: A cross-sectional study. Sci. Rep. 2020, 10, 16093. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, M.; Ren, Y.; Bi, J.; Du, Z.; Zhang, M.; Wang, Y.; Zhang, L.; Wu, Z.; Lv, Y.; et al. Serum Irisin Predicts Posthepatectomy Complications in Patients with Hepatocellular Carcinoma. Dis. Mark. 2019, 2019, 9850191. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 2020, 10, e173. [Google Scholar] [CrossRef]
- Ebert, T.; Focke, D.; Petroff, D.; Wurst, U.; Richter, J.; Bachmann, A.; Lössner, U.; Kralisch, S.; Kratzsch, J.; Beige, J.; et al. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur. J. Endocrinol. 2014, 170, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Deng, W. Association of Serum Irisin Concentrations with Presence and Severity of Coronary Artery Disease. Med. Sci. Monit. 2016, 22, 4193–4197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Gao, R.; Bei, Y.; Li, J.; Zhang, H.; Zhou, Y.; Yao, W.; Xu, D.; Zhou, F.; Jin, M.; et al. Serum Irisin Predicts Mortality Risk in Acute Heart Failure Patients. Cell Physiol. Biochem. 2017, 42, 615–622. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, M.; Wang, X.; Li, S.; Yang, D. The Role of Myokines and Adipokines in Hypertension and Hypertension-related Complications. Hypertens. Res. 2019, 42, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Z.; Qu, C.; Dong, Y. Effects of 12 Weeks Resistance Training on Serum Irisin in Older Male Adults. Front. Physiol. 2017, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto-Mikami, E.; Sato, K.; Kurihara, T.; Hasegawa, N.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE 2015, 10, e0120354. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.T.; Zhang, S.; Dubielecka, P.M.; Du, J.; Yano, N.; Chin, Y.E.; Zhuang, S.; Qin, G.; Zhao, T.C. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J. Cell Physiol. 2017, 232, 3775–3785. [Google Scholar] [CrossRef]
- Moscoso, I.; Cebro-Márquez, M.; Rodríguez-Mañero, M.; González-Juanatey, J.R.; Lage, R. FNDC5/Irisin counteracts lipotoxic-induced apoptosis in hypoxic H9c2 cells. J. Mol. Endocrinol. 2019, 63, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, F.; Zhang, Y.; Zhang, Y.; Wang, F.; Jiang, M.; Wang, Z.; Zhang, M.; Li, S.; Yang, L.; et al. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS ONE 2014, 9, e110273. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, F.; Zheng, Y.; Cheng, X.; Zhao, H. Irisin Ameliorates Hypoxia/Reoxygenation-Induced Inflammation and Apoptosis in PC12 Cells by Inhibiting TLR4/MYD88 Signaling Pathway. Curr. Top Nutraceutical Res. 2019, 17, 329–336. [Google Scholar]
- Yan, W.J.; Chen, Y.H.; Guo, Y.Z.; Xia, Y.L.; Li, C.Y.; Du, Y.H.; Lin, C.; Xu, X.M.; Qi, T.T.; Fan, M.M.; et al. Irisin Promotes Cardiac Homing of Intravenously Delivered MSCs and Protects against Ischemic Heart Injury. Adv. Sci. 2022, 9, 2103697. [Google Scholar] [CrossRef] [PubMed]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yan, S.; Luo, L.; Yang, L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol. Med. Rep. 2019, 19, 1074–1082. [Google Scholar] [CrossRef]
- Arazi, H.; Babaei, P.; Moghimi, M.; Asadi, A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021, 21, 50. [Google Scholar] [CrossRef]
- Belviranli, M.; Okudan, N.; Kabak, B.; Erdoğan, M.; Karanfilci, M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Physician Sportsmed. 2016, 44, 290–296. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Ribeiro, F.C.; Sudo, F.K.; Drummond, C.; Assunção, N.; Vanderborght, B.; Tovar-Moll, F.; Mattos, P.; De Felice, F.G.; Ferreira, S.T. Cerebrospinal fluid irisin correlates with amyloid-β, BDNF, and cognition in Alzheimer’s disease. Alzheimers Dement 2020, 12, e12034. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Badr Roomi, A.; Nori, W.; Mokram Hamed, R. Lower Serum Irisin Levels Are Associated with Increased Osteoporosis and Oxidative Stress in Postmenopausal. Rep. Biochem. Mol. Biol. 2021, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.N.; Alsharidah, A.S.; Mousa, A.M.; Edrees, H.M. Irisin Has a Protective Role against Osteoporosis in Ovariectomized Rats. Biomed. Res. Int. 2021, 2021, 5570229. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Nie, Y.; Li, S.; Roger, A.; Shen, X.; Yang, M.; et al. Irisin ameliorates bone loss in ovariectomized mice. Climacteric 2020, 23, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [Green Version]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef]
- Xu, L.; Shen, L.; Yu, X.; Li, P.; Wang, Q.; Li, C. Effects of irisin on osteoblast apoptosis and osteoporosis in postmenopausal osteoporosis rats through upregulating Nrf2 and inhibiting NLRP3 inflammasome. Exp. Ther. Med. 2020, 19, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Qiao, X.; Cai, Y.; Li, A.; Shan, D. Lower circulating irisin in middle-aged and older adults with osteoporosis: A systematic review and meta-analysis. Menopause 2019, 26, 1302–1310. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef]
- Guarnotta, V.; Prinzi, A.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Circulating Irisin Levels as a Marker of Osteosarcopenic-Obesity in Cushing’s Disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Ciresi, A.; Pizzolanti, G.; Guarnotta, V.; Giordano, C. Circulating Irisin Levels in Children With GH Deficiency Before and After 1 Year of GH Treatment. J. Clin. Endocrinol. Metab. 2019, 104, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Rank | Country | Number of Publications | Rank | Country | Centrality |
|---|---|---|---|---|---|
| 1 | Peoples R China | 376 | 1 | United States | 0.47 |
| 2 | United States | 232 | 2 | Spain | 0.26 |
| 3 | Turkey | 156 | 3 | England | 0.14 |
| 4 | Poland | 102 | 4 | Germany | 0.10 |
| 5 | Italy | 101 | 5 | Egypt | 0.10 |
| 6 | Iran | 85 | 6 | Australia | 0.09 |
| 7 | South Korea | 79 | 7 | Saudi Arabia | 0.09 |
| 8 | Germany | 71 | 8 | Italy | 0.07 |
| 9 | Spain | 68 | 9 | Turkey | 0.06 |
| 10 | Brazil | 54 | 10 | France | 0.06 |
| Rank | Institute | Country/Region | Number of Publications | Centrality |
|---|---|---|---|---|
| 1 | Firat University | Turkey | 54 | 0.01 |
| 2 | Harvard University | United States | 34 | 0.12 |
| 3 | Shandong University | China | 32 | 0.04 |
| 4 | Sichuan University | China | 20 | 0.14 |
| 5 | University of Bari Aldo Moro | Italy | 19 | 0.00 |
| 6 | Islamic Azad University | Iran | 19 | 0.02 |
| 7 | Harvard Medical School | United States | 19 | 0.09 |
| 8 | Gdansk University of Physical Education and Sport | Poland | 18 | 0.00 |
| 9 | Xi An Jiao Tong University | China | 18 | 0.03 |
| 10 | University Isfahan | Iran | 18 | 0.05 |
| Ranking | Journal | Country | Count | IF (2020) | JCR (2020) |
|---|---|---|---|---|---|
| 1 | PLoS ONE | United States | 46 | 3.240 | Q2 |
| 2 | International Journal of Molecular Sciences | United States | 34 | 5.924 | Q1/Q2 |
| 3 | Metabolism-Clinical and Experimental | Netherlands | 31 | 8.697 | Q1 |
| 4 | Scientific Reports | England | 29 | 4.380 | Q1 |
| 5 | Frontiers in Physiology | Switzerland | 27 | 4.566 | Q1 |
| 6 | Peptides | United States | 23 | 3.750 | Q2/Q3 |
| 7 | Journal of Clinical Endocrinology and Metabolism | United States | 22 | 5.799 | Q1 |
| 9 | Frontiers in Endocrinology | Switzerland | 17 | 5.555 | Q1 |
| 10 | Nutrients | Switzerland | 15 | 5.719 | Q1 |
| Ranking | Keywords | Occurrences | Total Link Strength | Ranking | Keywords | Occurrences | Total Link Strength |
|---|---|---|---|---|---|---|---|
| 1 | irisin | 970 | 5364 | 11 | fndc5 | 213 | 1439 |
| 2 | exercise | 491 | 3261 | 12 | serum | 171 | 1179 |
| 3 | obesity | 412 | 2791 | 13 | muscle | 169 | 1107 |
| 4 | skeletal muscle | 361 | 2402 | 14 | association | 163 | 1113 |
| 5 | adipose tissue | 301 | 2098 | 15 | metabolism | 140 | 902 |
| 6 | expression | 298 | 1838 | 16 | inflammation | 140 | 801 |
| 7 | fat | 292 | 1714 | 17 | plasma | 129 | 895 |
| 8 | circulating irisin | 258 | 1813 | 18 | physical activity | 115 | 763 |
| 9 | myokine | 248 | 1556 | 19 | humans | 110 | 829 |
| 10 | insulin resistance | 236 | 1578 | 20 | weight loss | 110 | 785 |
| Ranking | Frequency | Author | Title | Year | Journal | References |
|---|---|---|---|---|---|---|
| 1 | 937 | Boström, P. | A PGC1α a-dependent myokine that drives the browning of white fat and thermogenesis | 2012 | Nature | [1] |
| 2 | 472 | Huh, J.Y. | FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II mRNA expression and circulating concentrations in response to weight loss and exercise | 2012 | Metabolism-clinical and Experimental | [2] |
| 3 | 422 | Moreno-Navarrete, J.M. | Irisin is expressed and produced by Human muscle and adipose tissue in association with obesity and insulin resistance | 2013 | Journal of Clinical Endocrinology and Metabolism | [20] |
| 4 | 314 | Roca-Rivada, A. | FNDC5/Irisin is not only a myokine but also an adipokine | 2013 | PLoS ONE | [19] |
| 5 | 272 | Liu, J.J. | Lower circulating irisin is associated with type 2 diabetes mellitus. | 2013 | Journal of Diabetes and its Complications | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qi, B.; Gan, L.; Shen, Y.; Zou, Y. A Bibliometric Analysis of the Literature on Irisin from 2012–2021. Int. J. Environ. Res. Public Health 2022, 19, 6153. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19106153
Liu J, Qi B, Gan L, Shen Y, Zou Y. A Bibliometric Analysis of the Literature on Irisin from 2012–2021. International Journal of Environmental Research and Public Health. 2022; 19(10):6153. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19106153
Chicago/Turabian StyleLiu, Jiangshan, Bote Qi, Lin Gan, Yanli Shen, and Yu Zou. 2022. "A Bibliometric Analysis of the Literature on Irisin from 2012–2021" International Journal of Environmental Research and Public Health 19, no. 10: 6153. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph19106153