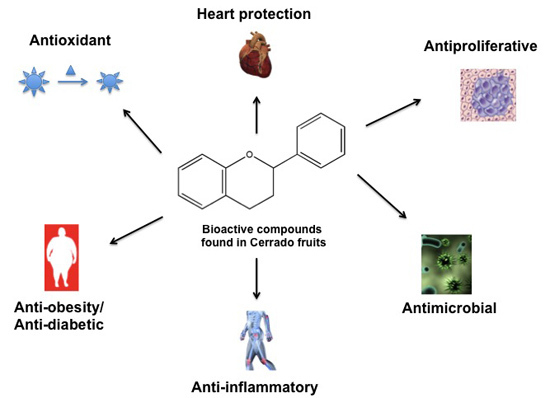
Bioactive Compounds Found in Brazilian Cerrado Fruits
Abstract
:1. Introduction
| Name (Scientific/Popular) | Main Metabolites a | Functional Properties |
|---|---|---|
| Anacardiaceae | ||
| Spondias mombin/cajá or taperebá | β-Cryptoxanthin (26) | Antioxidant |
| Annonaceae | ||
| Annona crassiflora/araticum | Ascorbic acid (17), caffeic acid (54), quinic acid (5), ferulic acid (55), xanthoxylin (56), and rutin (57) | Antioxidant |
| Arecaceae (Palmae) | ||
| Mauritia flexuosa/buriti | β-Carotene (25), α-carotene (24), lutein (41), and gallic acid (2) | Reverse clinical xerophthalmia and restore liver reserves of vitamin A |
| Caryocaraceae | ||
| Caryocar spp./pequi | Ethyl galate (1), gallic acid (2), methyl shikimate (3), lupeol (4), quinic acid (5), quercetin (6), and quercetin 3-O-arabinose (7), ethyl hexanoate (8), ethyl octanoate (9), β-ocimene (10), and hexanoic acid (11) | Antioxidant, antiaging, antiproliferative, and immunomodulatory |
| Leguminosae | ||
| Dipteryx alata/baru | Oleic, linoleic (12), linolenic (13), gadoleic (14), and erucic (15), phytic acid (16) | Antioxidant and cardiovascular diseases protection |
| Myrtaceae | ||
| Eugenia dysenterica/cagaita | Ascorbic acid (17), acetic acid (18), lactic acid (19), malic acid (20), succinic acid (21), tartaric acid (22), citric acid (23), α-carotene (24), β-carotene (25), β-cryptoxanthin (26) and lycopene, α- (27), β- (28), γ- (29) and δ-tocopherol (30), tocotrienol (31), tetrahydrofolate (32), 5-methyltetrahydrofolate (33), 5-formyltetrahydrofolate (34), and ellagic acid (35) | Laxative and anti-obesity |
| Eugenia uniflora/pitanga | Delphinidin-3-O-β-glucopyranoside (36), myricetin (37), cyanidin (38), quercetin (6), ellagic acid (35), and proanthocyanidins | Promising natural ingredient for food and nutraceutical manufacturers |
| Myrciaria cauliflora/jabuticaba | Cyanidin-3-O-glucoside (42), delphinidin-3-O-glucoside (36), gallic acid (2), ellagic acid (35), isoquercitrin (43), quercimeritrin (44), quercitrin (45), myricitrin (46), and quercetin (6) | Antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, could be used in chronic obstructive pulmonary disease (COPD) treatment |
| Psidiumguajava/goiaba | Ascorbic acid (17), myricetin (37), abscisic acid (47), and madecassic acid (48) | Antioxidant, antidiarrheal, antimicrobial, could reduce blood pressure and sugar, triglycerides and cholesterol blood levels, analgesic, and anti-inflammatory |
| Psidium spp./araçá | (−)-Epicatechin (49), gallic acid (2), taxifolin (50), quercetin (6), ellagic acid (35), all-trans-β-cryptoxanthin (51), β-carotene (25), and lutein (41) | Antimicrobial, antiproliferative, and could be involved in vasodilation |
| Rubiaceae | ||
| Genipa americana/jenipapo | Campesterol (38), stigmasterol (39), and β-sitosterol (40) | Anti-obesity, antioxidant, and antiproliferative |
| Sapotaceae | ||
| Hancornia speciosa/mangaba | β-Carotene (25), ascorbic acid (17), tocotrienol (31) and (6S)-5-formyl-5,6,7,8-tetrahydrofolate (5-FTHF) (34) | Antioxidant, antidiabetic, and anti-obesity |
| Solanaceae | ||
| Solanum lycocarpum/lobeira | Solamargine (52) and solasonine (53) | Antidiabetic, anti-inflammatory, and anticancer |




2. Caryocar brasiliense Camb.
3. Dipteryx alata Vog.
4. Eugenia spp.
5. Genipa americana L.
6. Hancornia speciosa Gomes
7. Mauritia flexuosa L.f.
8. Myrciaria cauliflora (DC) Berg
9. Psidium spp.
10. Solanum lycocarpum St. Hill
11. Spondias mombin L.
12. Other Cerrado Fruits
13. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, T. Development of functional foods. Biosci. Microbiota Food Health 2014, 33, 117–128. [Google Scholar] [CrossRef] [PubMed]
- WHO; IUCN; WWF. Guidelines on the Conservation of Medicinal Plants; Published by The International Union for Conservation of Nature and Natural Resources (IUCN): Gland, Switzerland; in partnership with The World Health Organization (WHO): Geneva, Switzerland; WWF–World Wide Fund for Nature: Gland, Switzerland, 1993; p. 38. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.; Freitas, A.F.R.; Pereira, M.S.V.; Oliveira, C.R.M.; Diniz, M.F.F.M.; Pessôa, H.L.F. Eficácia antifúngica dos extratos da Lippia sidoides Cham. e Matricaria recutita Linn. sobre leveduras do Gênero Candida. BioFar 2011, 5, 18–23. [Google Scholar]
- Sano, E.E.; Rosa, R.; Brito, J.L.; Ferreira, L.G. Land cover mapping of the tropical savanna region in Brazil. Environ. Monit. Assess. 2010, 166, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Fank-de-Carvalho, S.M.; Somavilla, N.S.; Marchioretto, M.S.; Báo, S.N. Plant structure in the Brazilian neotropical savannah species. In Biodiversity in Ecosystems—Linking Structure and Function; Lo, Y., Blanco, J.A., Roy, S., Eds.; InTech: Rijeka, Croatia, 2015; pp. 425–459. [Google Scholar]
- Beuchle, R.; Grecchi, R.C.; Shimabukuro, Y.E.; Seliger, R.; Eva, H.D.; Sano, E.; Achard, F. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl. Geogr. 2015, 58, 116–127. [Google Scholar] [CrossRef]
- Jepson, W. A disappearing biome? Reconsidering land cover change in the Brazilian savanna. Geogr. J. 2005, 17, 99–111. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian Cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Silva, J.F.; Farinas, M.R.; Felfili, J.M.; Klink, C.A. Spatial heterogeneity, land use and conservation in the Cerrado region of Brazil. J. Biogeogr. 2006, 33, 536–548. [Google Scholar] [CrossRef]
- Violante, I.M.; Hamerski, L.; Garcez, W.S.; Batista, A.L.; Chang, M.R.; Pott, V.J.; Garcez, F.R. Antimicrobial activity of some medicinal plants from the Cerrado of the centralwestern region of Brazil. Braz. J. Microbiol. 2012, 43, 1302–1308. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; Ramos, M.A.; Melo, J.G. New strategies for drug discovery in tropical forests based on ethnobotanical and chemical ecological studies. J. Ethnopharmacol. 2012, 140, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, O.R.; Kaplan, M.A.C.; Borin, M.R.M. Biodiversidade—Um Enfoque Químico-Biológico; Editora UFRJ: Rio de Janeiro, Brazil, 1996. [Google Scholar]
- Sun, Q.; Heilmann, J.; Konig, B. Natural phenolic metabolites with anti-angiogenic properties—A review from the chemical point of view. Beilstein J. Org. Chem. 2015, 11, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Frutas Nativas da Região Centro-Oste do Brasil; Vieira, R.F.; Costa, T.S.A.; Silva, D.B.; Ferreira, F.R.; Sano, S.M. (Eds.) Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2006.
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G.L. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Siqueira, E.M.; Rosa, F.R.; Fustinoni, A.M.; de Sant’Ana, L.P.; Arruda, S.F. Brazilian savanna fruits contain higher bioactive compounds content and higher antioxidant activity relative to the conventional red delicious apple. PLoS ONE 2013, 8, e72826. [Google Scholar] [CrossRef] [PubMed]
- Rocha, W.S.; Lopes, R.M.; Silva, D.B.; Vieira, R.F.; Silva, J.P.; Agostini-Costa, T.S. Total phenolics and condensed tannins in native fruits from brazilian savanna. Rev. Bras. Frutic. 2011, 33, 1215–1221. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [PubMed]
- Vieira, R.F.; Martins, M.V.M. Recursos genéticos de plantas medicinais de cerrado: Uma compilação de dados. Braz. J. Med. Plants 2000, 3, 13–36. [Google Scholar]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant activity of Caryocar brasiliense (pequi) and characterization of components by electrospray ionization mass spectrometry. Food Chem. 2008, 110, 711–717. [Google Scholar] [CrossRef]
- Geőcze, K.C.; Barbosa, L.C.A.; Fidêncio, P.H.; Silvério, F.O.; Lima, C.F.; Barbosa, M.C.A.; Ismail, F.M.D. Essential oils from pequi fruits from the Brazilian Cerrado ecosystem. Food Res. Int. 2013, 54, 1–8. [Google Scholar] [CrossRef]
- Damiani, C.; Vilas Boas, E.V.B.; Ferri, P.H.; Pinto, D.M.; Rodrigues, L.J. Volatile compounds profile of fresh-cut peki fruit stored under different temperatures. Ciênc. Tecnol. Aliment. 2009, 29, 435–439. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Silva, M.H.L. Aroma volatiles of pequi fruit (Caryocar brasiliense Camb.). J. Food Compos. Anal. 2008, 21, 574–576. [Google Scholar] [CrossRef]
- Dury-Brun, C.; Hirata, Y.; Guillard, V.; Ducruet, V.; Chalier, P.; Voilley, A. Ethyl hexanoate transfer in paper and plastic food packaging by sorption and permeation experiments. J. Food Eng. 2008, 89, 217–226. [Google Scholar] [CrossRef]
- Elsss, S.; Preston, C.; Hertzig, C.; Heckel, F.; Richling, E.; Schreier, P. Aroma profiles of pineapple fruit (Ananascomosus [L.] Merr.) and pineapple products. LWT 2005, 38, 263–274. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Delvaux, F.; Daenen, L.; Verachtert, H.; Delvaux, F.R. Aging characteristics of different beer types. Food Chem. 2007, 103, 404–412. [Google Scholar] [CrossRef]
- Han, S.Y.; Pan, Z.Y.; Huang, D.F.; Ueda, M.; Wang, X.N.; Lin, Y. Highly efficient synthesis of ethyl hexanoate catalyzed by CALB-displaying Saccharomyces cerevisiae whole-cells in non-aqueous phase. J. Mol. Catal. B Enzym. 2009, 59, 168–172. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 5th ed.; WILEY-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Miranda-Vilela, A.L.; Akimoto, A.K.; Alves, P.C.; Pereira, L.C.; Goncalves, C.A.; Klautau-Guimaraes, M.N.; Grisolia, C.K. Dietary carotenoid-rich pequi oil reduces plasma lipid peroxidation and DNA damage in runners and evidence for an association with MnSOD genetic variant-Val9Ala. Genet. Mol. Res. 2009, 8, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vilela, A.L.; Pereira, L.C.; Goncalves, C.A.; Grisolia, C.K. Pequi fruit (Caryocar brasiliense Camb.) pulp oil reduces exercise-induced inflammatory markers and blood pressure of male and female runners. Nutr. Res. 2009, 29, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.F.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Caryocar brasiliense supercritical CO2 extract possesses antimicrobial and antioxidant properties useful for personal care products. BMC Complement. Altern. Med. 2014, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vilela, A.L.; Alves, P.C.Z.; Akimoto, A.K.; Lordelo, G.S.; de Nazare Klautau-Guimarães, M.; Grisolia, C.K. Under increased hydrogen peroxide conditions, the antioxidant effects of pequi oil (Caryocar brasiliense Camb.) to decrease DNA damage in runners are influenced by sex, age and oxidative stress-related genetic polymorphisms. Free Radic. Antioxid. 2011, 1, 27–39. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Grisolia, C.K.; Longo, J.P.; Peixoto, R.C.; de Almeida, M.C.; Barbosa, L.C.; Roll, M.M.; Portilho, F.A.; Estevanato, L.L.; Bocca, A.L. Oil rich in carotenoids instead of vitamins C and E as a better option to reduce doxorubicin-induced damage to normal cells of Ehrlich tumor-bearing mice: Hematological, toxicological and histopathological evaluations. J. Nutr. Biochem. 2014, 25, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.C.M.F.; Nogueira, J.C.B.; Morais, E.; Kageyama, P.Y.; Murgel, J.M.T.; Zandarin, M.A. O cumbaru—Dipteryx alata Vog, Estudo de diferentes procedências e progênies. Bol. Téc. Inst. Florest. 1986, 40, 281–290. [Google Scholar]
- Oliveira Sousa, A.G.; Fernandes, D.C.; Alves, A.M.; de Freitas, J.B.; Naves, M.M.V. Nutritional quality and protein value of exotic almonds and nut from the Brazilian savanna compared to peanut. Food Res. Int. 2011, 44, 2319–2325. [Google Scholar] [CrossRef]
- Martins, D.T.O.; Lima, J.C.S.; Rao, V.S.N. The acetone soluble fraction from bark extract of Stryphnodendron adstringens (Mart.) coville inhibits gastric acid secretion and experimental gastric ulceration in rats. Phytother. Res. 2002, 16, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.S.; Borges, L.L.; Paula, J.R.; Conceição, E.C. Impact of different extraction methods on the quality of Dipteryx alata extracts. Rev. Bras. Farmacogn. 2013, 23, 521–526. [Google Scholar] [CrossRef]
- Bento, A.P.; Cominetti, C.; Simoes Filho, A.; Naves, M.M. Baru almond improves lipid profile in mildly hypercholesterolemic subjects: A randomized, controlled, crossover study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, E.M.A.; Marin, A.M.F.; da Cunha, M.S.B.; Fustinoni, A.M.; de Sant’Ana, L.P.; Arruda, S.F. Consumption of baru seeds (Dipteryx alata Vog.), a Brazilian savanna nut, prevents iron-induced oxidative stress in rats. Food Res. Int. 2012, 45, 427–433. [Google Scholar] [CrossRef]
- Genovese, M.I.; Silva Pinto, M.; Gonçalves, A.E.S.S.; Lajolo, F.M. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci. Technol. Int. 2008, 14, 207–214. [Google Scholar] [CrossRef]
- Schwan, R.F.; Mendonça, A.T.; Santos, J.J., Jr.; Rodrigues, V.; Wheals, A.E. Microbiology and physiology of cachaça (aguardente) fermentations. Antonie Van Leeuwenhoek 2001, 79, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.O.; Araújo, R.R.; Tacon, L.A.; Conceição, E.C.; Bara, M.T.F.; Paula, J.A.M.; Freitas, L.A.P. Development of a phytopharmaceutical intermediate product. Dry. Technol. 2011, 29, 709–718. [Google Scholar] [CrossRef]
- Cardoso, L.M.; Martino, H.S.D.; Moreira, A.V.B.; Ribeiro, S.M.R.; Pinheiro-Sant’Ana, H.M. Cagaita (Eugenia dysenterica DC.) of the Cerrado of Minas Gerais, Brazil: Physical and chemical characterization, carotenoids and vitamins. Food Res. Int. 2011, 44, 2151–2154. [Google Scholar] [CrossRef]
- Gonçalves, A.E.S.S.; Lajolo, F.M.; Genovese, M.I. Chemical composition and antioxidant/antidiabetic potential of Brazilian native fruits and commercial frozen pulps. J. Agric. Food Chem. 2010, 58, 4666–4674. [Google Scholar] [CrossRef] [PubMed]
- Palhares, D. Caracterizac¸ ão farmacognóstica das folhas de Eugenia dysenterica DC (Myrtaceae Jussieu). Rev. Lecta 2003, 21, 29–36. [Google Scholar]
- Lima, T.B.; Silva, O.N.; Oliveira, J.T.; Vasconcelos, I.M.; Scalabrin, F.B.; Rocha, T.L.; Grossi-de-Sa, M.F.; Silva, L.P.; Guadagnin, R.V.; Quirino, B.F.; et al. Identification of E. dysenterica laxative peptide: A novel strategy in the treatment of chronic constipation and irritable bowel syndrome. Peptides 2010, 31, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Donado-Pestana, C.M.; Belchior, T.; Genovese, M.I. Phenolic compounds from cagaita (Eugenia dysenterica DC.) fruit prevent body weight and fat mass gain induced by a high-fat, high-sucrose diet. Food Res. Int. 2015. [Google Scholar] [CrossRef]
- Correia, R.T.; Borges, K.C.; Medeiros, M.F.; Genovese, M.I. Bioactive compounds and phenolic-linked functionality of powdered tropical fruit residues. Food Sci. Technol. Int. 2012, 18, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonbulow, J.F.W.; Carmona, R.; Parente, T.V. Treatment and storage of Eugenia calycina seeds. Pesqui. Agropecu. Bras. 1994, 29, 961–970. [Google Scholar]
- Ono, M.; Ishimatsu, N.; Masuoka, C.; Yoshimitsu, H.; Tsuchihashi, R.; Okawa, M.; Kinjo, J.; Ikeda, T.; Nohara, T. Three new monoterpenoids from the fruit of Genipa americana. Chem. Pharm. Bull. 2007, 55, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.A.; Ballus, C.A.; Teixeira-Filho, J.; Godoy, H.T. Phytosterols and tocopherols content of pulps and nuts of Brazilian fruits. Food Res. Int. 2010, 43, 1603–1606. [Google Scholar] [CrossRef]
- Abidi, S.L. Chromatographic analysis of plant sterols in foods and vegetable oils. J. Chromatogr. A 2001, 935, 173–201. [Google Scholar] [CrossRef]
- Toivo, J.; Phillips, K.; Lampi, A.-M.; Piironen, V. Determination of sterols in foods: Recovery of free, esterified, and glycosidic sterols. J. Food Compos. Anal. 2001, 14, 631–643. [Google Scholar] [CrossRef]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of phytosterols in foods. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Omena, C.M.B.; Valentim, I.B.; Guedes, G.d.S.; Rabelo, L.A.; Mano, C.M.; Bechara, E.J.H.; Sawaya, A.C.H.F.; Trevisan, M.T.S.; da Costa, J.G.; Ferreira, R.C.S.; et al. Antioxidant, anti-acetylcholinesterase and cytotoxic activities of ethanol extracts of peel, pulp and seeds of exotic Brazilian fruits. Food Res. Int. 2012, 49, 334–344. [Google Scholar] [CrossRef]
- Conceição, A.O.; Rossi, M.H.; de Oliveira, F.F.; Takser, L.; Lafond, J. Genipa americana (Rubiaceae) fruit extract affects mitogen-activated protein kinase cell pathways in human trophoblast-derived BeWo cells: Implications for placental development. J. Med. Food 2011, 14, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.D.M.; Reis, B.D.L.; Oliveira, D.D.S.; Pinheiro-Sant’Ana, H.M. Mangaba (Hancornia speciosa Gomes) from the Brazilian Cerrado: Nutritional value, carotenoids and antioxidant vitamins. Fruits 2014, 69, 89–99. [Google Scholar] [CrossRef]
- Lima, D.M. Tabela Brasileira de Composição de Alimentos-TACO; Universidade Estadual de Campinas (UNICAMP): Campinas, Brazil, 2011. [Google Scholar]
- Rufino, M.; Fernandes, F.; Alves, R.; Debrito, E. Free radical-scavenging behaviour of some North-East Brazilian fruits in a DPPH system. Food Chem. 2009, 114, 693–695. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Yang, C.S.; Suh, N. Cancer prevention by different forms of tocopherols. Top. Curr. Chem. 2013, 329, 21–33. [Google Scholar] [PubMed]
- Picciano, M.F.; Yetley, E.A.; Coates, P.M.; McGuire, M.K. Update on folate and human health. Nutr. Today 2009, 44, 142–152. [Google Scholar] [CrossRef]
- Lorenzi, H.; Souza, H.M.; Costa, J.T.M.; Cerqueira, L.S.C.; Ferreira, E. Palmeiras Brasileiras e Exóticas Cultivadas; Instituto Plantarum: Nova Odessa, Brazil, 2004. [Google Scholar]
- Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Candido, T.L.; Silva, M.R.; Agostini-Costa, T.S. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Mariath, J.G.; Lima, M.C.; Santos, L.M. Vitamin A activity of buriti (Mauritia vinifera Mart) and its effectiveness in the treatment and prevention of xerophthalmia. Am. J. Clin. Nutr. 1989, 49, 849–853. [Google Scholar] [PubMed]
- Clerici, M.T.P.S.; Carvalho-Silva, L.B. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Res. Int. 2011, 44, 1658–1670. [Google Scholar] [CrossRef]
- Wu, S.B.A.; Dastmalchi, K.; Long, C.L.; Kennelly, E.J. Metabolite Profiling of Jaboticaba (Myrciaria cauliflora) and Other Dark-Colored Fruit Juices. J. Agric. Food Chem. 2012, 60, 7513–7525. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.S.; Oh, S.; Eun, J.-B.; Ahmed, M. Nutritional compositions and health promoting phytochemicals of camu-camu (Myrciaria dubia) fruit: A review. Food Res. Int. 2011, 44, 1728–1732. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Errante, G.; Zappia, G.; Dugo, G. Identification of anthocyanins in berries by narrow-bore high-performance liquid chromatography with electrospray ionization detection. J. Agric. Food Chem. 2001, 49, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.L.; Conceição, E.C.; Silveira, D. Active compounds and medicinal properties of Myrciaria genus. Food Chem. 2014, 153, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Marostica, M.R. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, K.; Flores, G.; Wu, S.B.; Ma, C.H.; Dabo, A.J.; Whalen, K.; Reynertson, K.A.; Foronjy, R.F.; D’Armiento, J.M.; Kennelly, E.J. Edible Myrciaria vexator fruits: Bioactive phenolics for potential COPD therapy. Bioorg. Med. Chem. 2012, 20, 4549–4555. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Hager, T.J.; Hager, A.; Howard, L. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.; Hager, A.; Wilkes, S.; Howard, L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol. Nutr. Food Res. 2009, 53, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Landrum, L.R.; Kawasaki, M.L. The Genera of Myrtaceae in Brazil: An Illustrated Synoptic Treatment and Identification Keys; Brittonia: Bronx, NY, USA, 1997. [Google Scholar]
- Hassimotto, N.M.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Gullon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Wu, S.B.; Negrin, A.; Kennelly, E.J. Chemical composition and antioxidant activity of seven cultivars of guava (Psidium guajava) fruits. Food Chem. 2015, 170, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Sanda, K.A.; Grema, H.A.; Geidam, Y.A.; Bukar-Kolo, Y.M. Pharmacological aspects of Psidium guajava: An update. Int. J. Pharmacol. 2011, 7, 316–324. [Google Scholar] [CrossRef]
- Coutiño, R.R.; Hernández, C.P.; Giles, R.H. Lectins in fruits having gastrointestinal activity: Their participation in the hemagglutinating property of Escherichia coli O157:H7. Arch. Med. Res. 2001, 32, 251–257. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [PubMed]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef]
- Hamacek, F.R.; Santos, P.R.G.; Cardoso, L.M.; Ribeiro, S.M.R.; Pinheiro-Sant’Ana, H.M. “Araçá of Cerrado” from the Brazilian savannah: Physical characteristics, chemical composition, and content of carotenoids and vitamins. Fruits 2013, 68, 467–481. [Google Scholar] [CrossRef]
- Raseira, M.C.B.; Raseira, A. Contribuição ao estudo do araçazeiro (Psidium cattleyanum); EMBRAPA/CPACT: Pelotas, Brazil, 1996. [Google Scholar]
- Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; Rosso, V.V. Phenolic compounds and carotenoids from four fruits native from the Brazilian Atlantic forest. J. Agric. Food Chem. 2014, 62, 5072–5284. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.R.; Martins, G.Z.; Magalhaes, N.O.; Almeida, A.E.; Pietro, R.C.; Silva, F.A.; Cicarelli, R.M. In vitro trypanocidal activity of solamargine and extracts from Solanum palinacanthum and Solanum lycocarpum of Brazilian Cerrado. An. Acad. Bras. Cienc. 2013, 85, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.; Kuehn, C.C.; Cardoso, J.F.; Oliveira, L.G.; Magalhaes, L.G.; Tiossi, R.F.; Rodrigues, V.; Zucolloto, S.; Prado, J.C., Jr.; McChesney, J.D.; et al. Immunomodulatory effect of the alkaloidic extract of Solanum lycocarpum fruits in mice infected with Schistosoma mansoni. Exp. Parasitol. 2013, 133, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Nakamura, S.; Ozaki, K.; Kumahara, A.; Morikawa, T.; Matsuda, H. Structures of steroidal alkaloid oligoglycosides, robeneosides A and B, and antidiabetogenic constituents from the Brazilian medicinal plant Solanum lycocarpum. J. Nat. Prod. 2007, 70, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Blankemeyer, J.T.; McWilliams, M.L.; Rayburn, J.R.; Weissenberg, M.; Friedman, M. Developmental toxicology of solamargine and solasonine glycoalkaloids in frog embryos. Food Chem. Toxicol. 1998, 36, 383–389. [Google Scholar] [CrossRef]
- Tiossi, R.F.; Da Costa, J.C.; Miranda, M.A.; Praca, F.S.; McChesney, J.D.; Bentley, M.V.; Bastos, J.K. In vitro and in vivo evaluation of the delivery of topical formulations containing glycoalkaloids of Solanum lycocarpum fruits. Eur. J. Pharm. Biopharm. 2014, 88, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Piassi, F.G.; Moyses, M.R.; Bazzolli, D.M.; Bissoli, N.D.S. Glycemic and urinary volume responses in diabetic mellitus rats treated with Solanum lycocarpum. Appl. Physiol. Nutr. Metab. 2010, 35, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.; Ferreira, P.M.; Matos, L.G.; Ferreira, E.C.; Rodovalho, W.; Ferri, P.H.; Ferreira, H.D.; Costa, E.A. Anti-infiammatory effect of Solanum lycocarpum fruits. Phytother. Res. 2003, 17, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.A.; Hobby, T.; Cipollini, M. Efficacy and mechanisms of α-solasonine-and α-solamargine-induced cytolysis on two strains of Trypanosoma cruzi. J. Chem. Ecol. 2006, 32, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.; Magalhaes, L.G.; Tiossi, R.F.; Kuehn, C.C.; Oliveira, L.G.; Rodrigues, V.; McChesney, J.D.; Bastos, J.K. Evaluation of the schistosomicidal activity of the steroidal alkaloids from Solanum lycocarpum fruits. Parasitol. Res. 2012, 111, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chataing, B.; Buitrago, C.N.; Concepcion, J.L.; Usubillaga, A. Estudio clínico de la efectividad de extractos alcaloideos obtenidos de los frutos del Solanun americanum Miller sobre el herpes simplex herpes zoster y herpes genitalis. Rev. Fac. Farm. 1996, 32, 15–28. [Google Scholar]
- Pinto, F.C.L.; Uchoa, D.E.A.; Silveira, E.R.; Pessoa, O.D.L.; Braz-Filho, R.; Silva, F.M.; Theodoro, P.N.E.T.; Espíndola, L.S. Antifungal glycoalkaloids, flavonoids and other chemical constituents of Solanum asperum. Quim. Nova 2011, 34, 284–288. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G.; Weissenberg, M. Interactions between the glycoalkaloids solasonine and solamargine in relation to inhibition of fungal growth. Phytochemistry 1994, 37, 1007–1011. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kozukue, N.; Han, J.-S.; Park, J.-H.; Chang, E.-Y.; Baek, E.-J.; Chang, J.-S.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.-W.; Hsu, S.-H.; Li, Y.-P.; Lin, W.-L.; Liu, L.-F.; Chang, L.-C.; Lin, C.-C.; Lin, C.-N.; Sheu, H.-M. Anticancer activity evaluation of the Solanum glycoalkaloid solamargine: Triggering apoptosis in human hepatoma cells. Biochem. Pharmacol. 2000, 60, 1865–1873. [Google Scholar] [CrossRef]
- Daunter, B.; Cham, B.E. Solasodine glycosides. In vitro preferential cytotoxicity for human cancer cells. Cancer Lett. 1990, 55, 209–220. [Google Scholar] [PubMed]
- Munari, C.; de Oliveira, P.; Campos, J.; Martins, S.; Da Costa, J.; Bastos, J.; Tavares, D. Antiproliferative activity of Solanum lycocarpum alkaloidic extract and their constituents, solamargine and solasonine, in tumor cell lines. J. Nat. Med. 2014, 68, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, S.; Cook, L.J.; Kersey, P.; Marks, R.; Cerio, R. Solasodine glycoalkaloids: A novel topical therapy for basal cell carcinoma. A double-blind, randomized, placebo-controlled, parallel group, multicenter study. Int. J. Dermatol. 2008, 47, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bosco, J.; Soares, K.T.; Aguiar-Filho, S.P.; Barros, R.V. A Cultura da Cajazeira; EMEPA-PB: João Pessoa, Brazil, 2000. [Google Scholar]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef]
- Wang, S.; Melnyk, J.P.; Tsao, R.; Marcone, M.F. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Roesler, R.; Malta, L.G.; Carrasco, L.C.; Holanda, R.B.; Sousa, C.A.S.; Pastore, G.M. Antioxidant activity of cerrado fruits. Ciênc. Tecnol. Aliment. 2007, 27, 53–60. [Google Scholar] [CrossRef]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant activity of Annona crassiflora: Characterization of major components by electrospray ionization mass spectrometry. Food Chem. 2007, 104, 1048–1054. [Google Scholar] [CrossRef]
- Roesler, R.; Malta, L.G.; Carrasco, L.C.; Pastore, G. Evaluation of the antioxidant properties of the Brazilian Cerrado fruit Annona crassiflora (Araticum). J. Food Sci. 2006, 71, C102–C107. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Proximate composition of guariroba (Syagrus oleracea), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata) palm fruits. Food Res. Int. 2011, 44, 2139–2142. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [Green Version]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailão, E.F.L.C.; Devilla, I.A.; Da Conceição, E.C.; Borges, L.L. Bioactive Compounds Found in Brazilian Cerrado Fruits. Int. J. Mol. Sci. 2015, 16, 23760-23783. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161023760
Bailão EFLC, Devilla IA, Da Conceição EC, Borges LL. Bioactive Compounds Found in Brazilian Cerrado Fruits. International Journal of Molecular Sciences. 2015; 16(10):23760-23783. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161023760
Chicago/Turabian StyleBailão, Elisa Flávia Luiz Cardoso, Ivano Alessandro Devilla, Edemilson Cardoso Da Conceição, and Leonardo Luiz Borges. 2015. "Bioactive Compounds Found in Brazilian Cerrado Fruits" International Journal of Molecular Sciences 16, no. 10: 23760-23783. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161023760