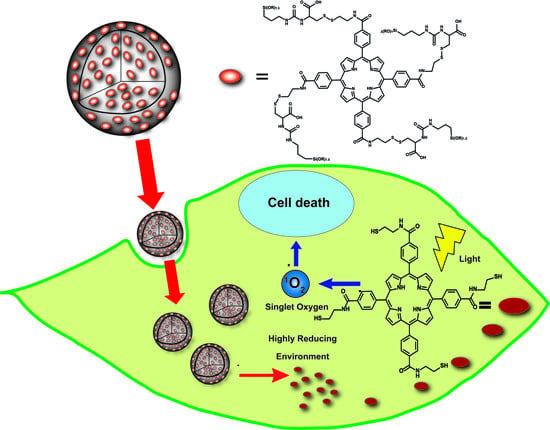
Redox-Responsive Porphyrin-Based Polysilsesquioxane Nanoparticles for Photodynamic Therapy of Cancer Cells
Abstract
:1. Introduction


2. Results and Discussion
2.1. Synthesis and Characterization of Redox-Responsive Tetrakis(Carboxyphenyl) Porphyrin (RR-TCPP) and Control Tetrakis(Carboxyphenyl) Porphyrin(C-TCPP) Silane Derivatives

2.2. Singlet Oxygen Generation of TCPP-Serine (4) and TCPP-EtSH (9)

2.3. Synthesis and Structural Characterization of RR-TCPP-PSilQ and C-TCPP-PSilQ Nanoparticles

| Sample | Diameter (nm) * n = 3 | PDI | ζ-Potential (mV) * n = 3 | Aromatic Content (%) | Loading of TCPP (µmol/g) |
|---|---|---|---|---|---|
| C-TCPP-PSilQNPs | 183.8 ± 10.5 | 0.39 | −39.7 ± 2.8 | 10.1 | 127.7 |
| RR-TCPP-PSilQNPs | 144.3 ± 15.0 | 0.33 | −44.5 ± 2.5 | 11.3 | 142.0 |
2.4. Photophysical and Photochemical Properties of C-TCPP- and RR-TCPP-PSilQ Nanoparticles

2.5. Stimuli-Responsive Properties of RR-TCPP-PSilQ Nanoparticles

2.6. In Vitro Phototoxicity of C-TCPP- and RR-TCPP-PSilQ Nanoparticles

3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of 5,10,15,20-Tetrakis(carbomethoxy)phenyl Porphyrin (TCM4PP) (1)
3.3. Synthesis of Tetrakis(carboxy)phenyl Porphyrin (TCPP) (2)
3.4. Synthesis of Succinimide Ester of TCPP (TCPP-SE) (3)
3.5. Synthesis of TCPP Serine Derivative (TCPP-Serine) (4)
3.6. Synthesis of Control TCPP Silane Derivative (C-TCPP) (5)
3.7. Synthesis of TCPP-Pyridine Disulfide Cysteamine (TCPP-PDSCA) (6)
3.8. Synthesis of TCPP-Cysteine Disulfide (TCPP-Cysteine) (7)
3.9. Synthesis of Redox-Responsive TCPP Silane Derivative (RR-TCPP) (8)
3.10. Synthesis of TCPP-Ethyl Thiol (TCPP-EtSH) by Reduction of TCPP-PDSCA with dl-Dithiothreitol (DTT) (9)
3.11. Synthesis of 2 Pyridyl Disulfide Cysteamine (PDSCA) (10)
3.12. Singlet Oxygen (1O2) Determination for TCPP (2), TCPP-Serine (4) and TCPP-EtSH (9)
3.13. Synthesis of C-TCPP- and RR-TCPP-PSilQ Nanoparticles
3.14. Singlet Oxygen (1O2) Determination for C-TCPP- and RR-TCPP-PSilQ Nanoparticles
3.15. Photophysical Characterization of C-TCPP- and RR-TCPP-PSilQ Nanoparticles
3.16. Release Profile of TCPP-EtSH from RR-TCPP-PSilQNPs under High Reducing Environment
3.17. In Vitro Phototoxicity of C-TCPP- and RR-TCPP-PSilQ Nanoparticles in Human Cervical Cancer (HeLa) Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaen, P.; Sanz-Rodriguez, F.; Cuevas, J.; Gonzalez, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Bagnato, V.S.; Cuenca, R.; Downie, G.H.; Sibata, C.H. The future of photodynamic therapy in oncology. Future Oncol. 2006, 2, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.-L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Chouikrat, R.; Seve, A.; Vanderesse, R.; Benachour, H.; Barberi-Heyob, M.; Richeter, S.; Raehm, L.; Durand, J.O.; Verelst, M.; Frochot, C. Non polymeric nanoparticles for photodynamic therapy applications: Recent developments. Curr. Med. Chem. 2012, 19, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zhang, S.; Yin, C.; Lin, G.; Li, Q. Designing nanoparticle carriers for enhanced drug efficacy in photodynamic therapy. Biomater. Sci. 2014, 2, 827–832. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Dai, T.; Chung, H.; Yaroslavsky, A.; Garcia-Diaz, M.; Chang, J.; Chiang, L.Y.; Hamblin, M.R. Can nanotechnology potentiate photodynamic therapy? Nanotechnol. Rev. 2012, 1, 111–146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Avci, P.; Sadasivam, M.; Chandran, R.; Parizotto, N.; Vecchio, D.; de Melo Wanessa, C.M.A.; Dai, T.; Chiang Long, Y.; Hamblin Michael, R. Shining light on nanotechnology to help repair and regeneration. Biotechnol. Adv. 2013, 31, 607–631. [Google Scholar] [CrossRef] [PubMed]
- Master, A.; Livingston, M.; Sen Gupta, A. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Sibani, S.A.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Photosensitiser delivery for photodynamic therapy. Part 2: Systemic carrier platforms. Expert Opin. Drug Deliv. 2008, 5, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Voon, S.H.; Kiew, L.V.; Lee, H.B.; Lim, S.H.; Noordin, M.I.; Kamkaew, A.; Burgess, K.; Chung, L.Y. In vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small 2014, 10, 4993–5013. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gong, H.; Qian, X.; Tan, P.; Li, Z.; Liu, T.; Liu, J.; Li, Y.; Liu, Z. Mesoporous silica nanorods intrinsically doped with photosensitizers as a multifunctional drug carrier for combination therapy of cancer. Nano Res. 2015, 8, 751–764. [Google Scholar] [CrossRef]
- Fan, W.; Shen, B.; Bu, W.; Chen, F.; He, Q.; Zhao, K.; Zhang, S.; Zhou, L.; Peng, W.; Xiao, Q.; et al. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous MR/UCL imaging. Biomaterials 2014, 35, 8992–9002. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Kori, T.; Ishimura, K. Photostable iodinated silica/porphyrin hybrid nanoparticles with heavy-atom effect for wide-field photodynamic/photothermal therapy using single light source. Adv. Funct. Mater. 2014, 24, 503–513. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Elnagheeb, M. Mesoporous silica nanoparticles loaded with cisplatin and phthalocyanine for combination chemotherapy and photodynamic therapy in vitro. Nanomaterials 2015, 5, 2302–2316. [Google Scholar] [CrossRef]
- Lovell, J.F.; Chen, J.; Jarvi, M.T.; Cao, W.-G.; Allen, A.D.; Liu, Y.; Tidwell, T.T.; Wilson, B.C.; Zheng, G. Fret quenching of photosensitizer singlet oxygen generation. J. Phys. Chem. B 2009, 113, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.K.; Lou, X.; Chen, Y.-C.; Koo Lee, Y.-E.; Yoon, E.; Kopelman, R. Nanophotosensitizers engineered to generate a tunable mix of reactive oxygen species, for optimizing photodynamic therapy, using a microfluidic device. Chem. Mater. 2014, 26, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Min, Y.; Hu, Q.; Xing, B.; Liu, B. NIR photoregulated chemo- and photodynamic cancer therapy based on conjugated polyelectrolyte-drug conjugate encapsulated upconversion nanoparticles. Nanoscale 2014, 6, 11259–11272. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Koo, H.; Lee, D.-E.; Min, S.; Lee, S.; Chen, X.; Choi, Y.; Leary, J.F.; Park, K.; Jeong, S.Y.; et al. Tumor-homing photosensitizer-conjugated glycol chitosan nanoparticles for synchronous photodynamic imaging and therapy based on cellular on/off system. Biomaterials 2011, 32, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Preuss, A.; Chen, K.; Hackbarth, S.; Wacker, M.; Langer, K.; Roeder, B. Photosensitizer loaded HSA nanoparticles II: In vitro investigations. Int. J. Pharm. 2011, 404, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Preuss, A.; Hackbarth, S.; Wacker, M.; Langer, K.; Roeder, B. Novel photosensitizer-protein nanoparticles for photodynamic therapy: Photophysical characterization and in vitro investigations. J. Photochem. Photobiol. B Biol. 2009, 96, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Ungaro, F.; Maglio, G.; Tirino, P.; Siracusano, G.; Sciortino, M.T.; Leone, N.; Palma, G.; Barbieri, A.; Arra, C.; et al. Biodegradable core-shell nanoassemblies for the delivery of docetaxel and Zn(II)-phthalocyanine inspired by combination therapy for cancer. J. Control. Release 2013, 167, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-H.; Min, H.S.; Li, L.; Tran, T.H.; Lee, Y.-K.; Kwon, I.C.; Choi, K.; Kim, K.; Huh, K.M. Cancer cell-specific photoactivity of pheophorbide a-glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 2013, 34, 6454–6463. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Mauriello-Jimenez, C.; Maynadier, M.; Cattoen, X.; Wong Chi Man, M.; Raehm, L.; Mongin, O.; Blanchard-Desce, M.; Garcia, M.; Gary-Bobo, M.; et al. Synthesis of disulfide-based biodegradable bridged silsesquioxane nanoparticles for two-photon imaging and therapy of cancer cells. Chem. Commun. (Camb. UK) 2015, 51, 12324–12327. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; DeCillis, D.; Fritts, L.; Vega, D.L. Porphyrin-based polysilsesquioxane nanoparticles to improve photodynamic therapy for cancer treatment. Proc. SPIE 2014, 8931. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Vega, D.L. Stimuli-responsive protoporphyrin IX silica-based nanoparticles for photodynamic therapy in vitro. RSC Adv. 2014, 4, 14400–14407. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Bruckner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Dolphin, D. (Ed.) The Porphyrins, Vol. 3: Physical Chemistry, Pt. A; Academic Press: New York, NY, USA, 1978; p. 636.
- Ha, J.-H.; Ko, S.; Lee, C.-H.; Lee, W.-Y.; Kim, Y.-R. Effect of core atom modification on photophysical properties and singlet oxygen generation efficiencies: Tetraphenylporphyrin analogues core-modified by oxygen and/or sulfur. Chem. Phys. Lett. 2001, 349, 271–278. [Google Scholar] [CrossRef]
- Marin, D.M.; Payerpaj, S.; Collier, G.S.; Ortiz, A.L.; Singh, G.; Jones, M.; Walter, M.G. Efficient intersystem crossing using singly halogenated carbomethoxyphenyl porphyrins measured using delayed fluorescence, chemical quenching, and singlet oxygen emission. Phys. Chem. Chem. Phys. 2015, 17, 29090–29096. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Huxford, R.C.; Lin, W. Hybrid nanomaterials for biomedical applications. Chem. Commun. 2010, 46, 5832–5849. [Google Scholar] [CrossRef] [PubMed]
- Khiterer, M.; Shea, K.J. Spherical, monodisperse, functional bridged polysilsesquioxane nanoparticles. Nano Lett. 2007, 7, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, D.L.; Lodge, P.; Vivero-Escoto, J.L. Redox-Responsive Porphyrin-Based Polysilsesquioxane Nanoparticles for Photodynamic Therapy of Cancer Cells. Int. J. Mol. Sci. 2016, 17, 56. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010056
Vega DL, Lodge P, Vivero-Escoto JL. Redox-Responsive Porphyrin-Based Polysilsesquioxane Nanoparticles for Photodynamic Therapy of Cancer Cells. International Journal of Molecular Sciences. 2016; 17(1):56. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010056
Chicago/Turabian StyleVega, Daniel L., Patrick Lodge, and Juan L. Vivero-Escoto. 2016. "Redox-Responsive Porphyrin-Based Polysilsesquioxane Nanoparticles for Photodynamic Therapy of Cancer Cells" International Journal of Molecular Sciences 17, no. 1: 56. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010056