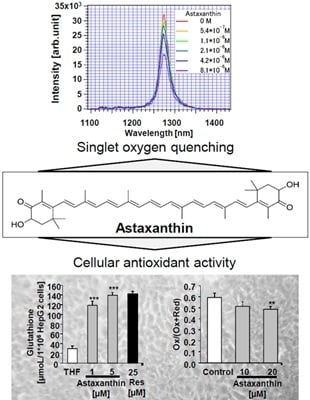
Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin
Abstract
:1. Introduction

2. Results and Discussion




| Gene | Astaxanthin [µM] | |
|---|---|---|
| 1 | 20 | |
| Gclc | 0.91 ± 0.08 | 1.03 ± 0.26 |
| Gsta | 1.01 ± 0.15 | 0.99 ± 0.18 |
| Gpx1 | 1.34 ± 0.24 | 1.12 ± 0.30 |
| Hmxo1 | 0.40 ± 0.04 | 0.85 ± 0.32 |
| Nqo1 | 1.21 ± 0.18 | 1.32 ± 0.21 |
| Sod1 | 1.22 ± 0.21 | 0.99 ± 0.18 |
| Sod2 | 1.35 ± 0.18 | 1.39 ± 0.54 |

3. Experimental Section
3.1. Radical Scavenging Measured by Electron Spin Resonance Spectroscopy (ESR)
3.2. Inhibition of Xanthine Oxidase
3.3. Quenching Experiments of Near-Infrared Emission Spectra of 1O2 by Astaxanthin
3.4. Cell Culture
3.5. Neutral Red Cell Viability Assay
3.6. Paraoxonase Activity
3.7. Glutathione Assay
3.8. Nrf2 Transactivation
3.9. RNA Isolation and qRT-PCR
3.10. Lipid Peroxidation Determined by BODIPY Assay
3.11. Statistical Analysis
| Gene | Gene-ID | Description | Primer, Forward (5′–3′) | Primer, Reverse (5′–3′) | Annealing (°C) |
|---|---|---|---|---|---|
| Gpx1 | 2876 | glutathione peroxidase 1 | ACACCCAGATGAACGAGCTG | CCGGACGTACTTGAGGGAAT | 58 |
| Sod1 | 6647 | superoxide dismutase 1, soluble | GGGGAAGCATTAAAGGACTG | CAACATGCCTCTCTTCATCC | 55 |
| Sod2 | 6648 | superoxide dismutase 2, mitochondrial | GCACTAGCAGCATGTTGAGC | GATCTGCGCGTTGATGTG | 55 |
| Hmox1 | 3162 | heme oxygenase 1 | CCA GGC AGA GAA TGC TGA GT | GTA GAC AGG GGC GAA GAC TG | 59 |
| Nqo1 | 1728 | NAD(P)H dehydrogenase [quinone] 1 | CTG ATC GTA CTG GCT CAC TC | GAA CAG ACT CGG CAG GAT AC | 58 |
| Gclc | 2729 | glutamate-cysteine ligase, catalytic subunit | TTT GGT CAG GGA GTT TCC AG | TGA ACA GGC CAT GTC AAC TG | 59 |
| Gst | 2938 | glutathione S-transferase alpha 1 | CGTATGTCCACCTGAGGAAA | GCCAACAAGGTAGTCTTGTCC | 60 |
| Gapdh | 2597 | glyceraldehyde-3-phosphate dehydrogenase | CAATGACCCCTTCATTGACC | GATCTCGCTCCTGGAAGATG | 58 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Hui, J.P.M.; Burton, I.W.; Thibault, M.-H.; Pelletier, C.; Boudreau, J.; Tchoukanova, N.; Subramanian, B.; Djaoued, Y.; Ewart, S.; et al. Characterization of shrimp oil from Pandalus borealis by high performance liquid chromatography and high resolution mass spectrometry. Mar. Drugs 2015, 13, 3849–3876. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Marin, D.P.; Bolin, A.P.; de Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C.; Guerra, B.A.; Leite, J.R.; Barros, M.P.; Mattei, R. Combined fish oil and astaxanthin supplementation modulates rat lymphocyte function. Eur. J. Nutr. 2012, 51, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Megdal, P.A.; Craft, N.A.; Handelman, G.J. A simplified method to distinguish farmed (Salmo salar) from wild salmon: Fatty acid ratios versus astaxanthin chiral isomers. Lipids 2009, 44, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Widmer, E.; Zell, R.; Broger, E.A.; Crameri, Y.; Wagner, H.P.; Dinkel, J.; Schlageter, M.; Lukáč, T. Technische Verfahren zur Synthese von Carotinoiden und verwandten Verbindungen aus 6-Oxo-isophoron. II. Ein neues Konzept für die Synthese von (3RS, 3´RS)-Astaxanthin. Helv. Chim. Acta 1981, 64, 2436–2446. (In German) [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sarada, R.; Shylaja, M.D.; Ravishankar, G.A. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Technol. 2015, 52, 6703–6710. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Yang, A.Y.; Guo, Y.; Kong, A.N.T. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta 2013, 1830, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Bai, S.-K.; Lee, K.-S.; Namkoong, S.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Yim, S.-V.; Chang, K.; Kwon, Y.-G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing IκB kinase-dependent NF-κB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [PubMed]
- Polotow, T.G.; Vardaris, C.V.; Mihaliuc, A.R.; Gonçalves, M.S.; Pereira, B.; Ganini, D.; Barros, M.P. Astaxanthin supplementation delays physical exhaustion and prevents redox imbalances in plasma and soleus muscles of Wistar rats. Nutrients 2014, 6, 5819–5838. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, Y.; Ishikawa, M.; Yoshioka, D.; Endoh, N.; Oowada, S.; Shimmei, M.; Fujii, H.; Kotake, Y. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with the ORAC-EPR method. J. Clin. Biochem. Nutr. 2012, 50, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstractions. 1. The reactions of phenols with 2,2-diphenyl-1-picrylhydrazyl (dpph*) in alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003, 523–524, 9–20. [Google Scholar] [CrossRef]
- Rimbach, G.; Höhler, D.; Fischer, A.; Roy, S.; Virgili, F.; Pallauf, J.; Packer, L. Methods to assess free radicals and oxidative stress in biological systems. Arch. Tierernahr. 1999, 52, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 694305. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Papa, T.B.R.; Pinho, V.D.; do Nascimento, E.S.P.; Santos, W.G.; Burtoloso, A.C.B.; Skibsted, L.H.; Cardoso, D.R. Astaxanthin diferulate as a bifunctional antioxidant. Free Radic. Res. 2015, 49, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Bjerkeng, B. Intraperitoneal and dietary administration of astaxanthin in rainbow trout (Oncorhynchus mykiss)—Plasma uptake and tissue distribution of geometrical E/Z isomers. Comp. Biochem. Physiol. B 2007, 147, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Zatloukalová, L.; Filipskỳ, T.; Hrdina, R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radic. Biol. Med. 2010, 49, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Moini, H.; Guo, Q.; Packer, L. Xanthine oxidase and xanthine dehydrogenase inhibition by the procyanidin-rich French maritime pine bark extract, pycnogenol: A protein binding effect. Adv. Exp. Med. Biol. 2002, 505, 141–149. [Google Scholar] [PubMed]
- Boesch-Saadatmandi, C.; Rimbach, G.; Jungblut, A.; Frank, J. Comparison of tetrahydrofuran, fetal calf serum, and Tween 40 for the delivery of astaxanthin and canthaxanthin to HepG2 cells. Cytotechnology 2011, 63, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Briviba, K.; Bornemann, R.; Lemmer, U. Visualization of astaxanthin localization in HT29 human colon adenocarcinoma cells by combined confocal resonance Raman and fluorescence microspectroscopy. Mol. Nutr. Food Res. 2006, 50, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Rimbach, G. Determinants of paraoxonase 1 status: Genes, drugs and nutrition. Curr. Med. Chem. 2011, 18, 5624–5643. [Google Scholar] [CrossRef] [PubMed]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother. Res. 2013, 27, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2014, 12, 145–152. [Google Scholar] [CrossRef]
- Morales, A.; García-Ruiz, C.; Miranda, M.; Marí, M.; Colell, A.; Ardite, E.; Fernández-Checa, J.C. Tumor necrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chain of γ-glutamylcysteine synthetase. J. Biol. Chem. 1997, 272, 30371–30379. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Miranda, M.; Sanchez-Reyes, A.; Colell, A.; Biete, A.; Fernández-Checa, J.C. Transcriptional regulation of the heavy subunit chain of γ-glutamylcysteine synthetase by ionizing radiation. FEBS Lett. 1998, 427, 15–20. [Google Scholar] [CrossRef]
- Moffat, G.J.; McLaren, A.W.; Wolf, C.R. Sp1-mediated transcriptional activation of the human Pi class glutathione S-transferase promoter. J. Biol. Chem. 1996, 271, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhang, X.; Huang, B.; Zhu, Y.; Chen, X. Astaxanthin suppresses MPP+-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar. Drugs 2013, 11, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.; Husberg, C.; Murphy, P.; Nordström, O.; Blomhoff, R.; Moskaug, J.O.; Kolstø, A.B. TCF11/Nrf1 overexpression increases the intracellular glutathione level and can transactivate the γ-glutamylcysteine synthetase (GCS) heavy subunit promoter. Biochim. Biophys. Acta 2001, 1517, 212–219. [Google Scholar] [CrossRef]
- Yang, H.; Magilnick, N.; Lee, C.; Kalmaz, D.; Ou, X.; Chan, J.Y.; Lu, S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-κB and AP-1. Mol. Cell. Biol. 2005, 25, 5933–5946. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, R.; Kojima, S. Increase of intracellular glutathione by low-level NO mediated by transcription factor NF-κB in RAW 264.7 cells. Biochim. Biophys. Acta 2005, 1744, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lababpour, A.; Lee, C.-G. Simultaneous measurement of chlorophyll and astaxanthin in Haematococcus pluvialis cells by first-order derivative ultraviolet-visible spectrophotometry. J. Biosci. Bioeng. 2006, 101, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nakanishi, Y.; Nakahara, M.; Wada, N.; Moro-oka, Y. Radical on structure effect antioxidant activity of catecholamines toward singlet oxygen and other reactive oxygen species in vitro. J. Clin. Biochem. Nutr. 2010, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Egert, S.; Schrader, C.; Coumoul, X.; Barouki, R.; Muller, M.J.; Wolffram, S.; Rimbach, G. Effect of quercetin on paraoxonase 1 activity-studies in cultured cells, mice and humans. J. Physiol. Pharmacol. 2010, 61, 99–105. [Google Scholar] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spinresonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, C.; Guizon, I.; Genestie-Denis, I.; Vannier, B.; Lorenzon, G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: Performance study of a new miniaturized protocol. Cell Biol. Toxicol. 1994, 10, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Ernst, I.; Iori, R.; Desel, C.; Rimbach, G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp. Dermatol. 2010, 19, 137–144. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010103
Dose J, Matsugo S, Yokokawa H, Koshida Y, Okazaki S, Seidel U, Eggersdorfer M, Rimbach G, Esatbeyoglu T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. International Journal of Molecular Sciences. 2016; 17(1):103. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010103
Chicago/Turabian StyleDose, Janina, Seiichi Matsugo, Haruka Yokokawa, Yutaro Koshida, Shigetoshi Okazaki, Ulrike Seidel, Manfred Eggersdorfer, Gerald Rimbach, and Tuba Esatbeyoglu. 2016. "Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin" International Journal of Molecular Sciences 17, no. 1: 103. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010103