Urine Aquaporin-2: A Promising Marker of Response to the Arginine Vasopressin Type-2 Antagonist, Tolvaptan in Patients with Congestive Heart Failure
Abstract
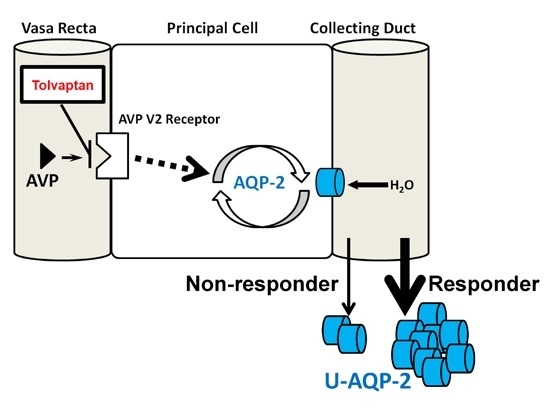
:1. Aquaporin-2 in Patients with Congestive Heart Failure (HF)

2. Tolvaptan and Its Responsiveness
3. Tolvaptan and Aquaporin-2

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HF | heart failure |
| AVP | arginine vasopressin |
| U-OSM | urine osmolality |
References
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kwon, T.H. A minireview on vasopressin-regulated aquaporin-2 in kidney collecting duct cells. Electrol. Blood Press. 2015, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.E. Hyponatremia associated with heart failure: Pathological role of vasopressin-dependent impaired water excretion. J. Clin. Med. 2015, 4, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Saito, T.; Fukagawa, A.; Higashiyama, M.; Nakamura, T.; Kusaka, I.; Nagasaka, S.; Honda, K.; Saito, T. Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antidiuresis in hyponatremia in elderly subjects. J. Clin. Endocrinol. Metab. 2001, 86, 1665–1671. [Google Scholar] [CrossRef]
- Robertson, G.L.; Mahr, E.A.; Athar, S.; Sinha, T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J. Clin. Investig. 1973, 52, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Rai, T.; Sekine, K.; Kanno, K.; Hata, K.; Miura, M.; Mizushima, A.; Marumo, F.; Sasaki, S. Urinary excretion of aquaporin-2 water channel protein in human and rat. J. Am. Soc. Nephrol. 1997, 8, 1357–1362. [Google Scholar] [PubMed]
- Elliot, S.; Goldsmith, P.; Knepper, M.; Haughey, M.; Olson, B. Urinary excretion of aquaporin-2 in humans: A potential marker of collecting duct responsiveness to vasopressin. J. Am. Soc. Nephrol. 1996, 7, 403–409. [Google Scholar] [PubMed]
- Umenishi, F.; Summer, S.N.; Cadnapaphornchai, M.; Schrier, R.W. Comparison of three methods to quantify urinary aquaporin-2 protein. Kidney Int. 2002, 62, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Ohmoto, Y.; Mori, T.; Iwata, F.; Muraguchi, M. Daily variance of urinary excretion of AQP2 determined by sandwich elisa method. Clin. Exp. Nephrol. 2012, 16, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Buemi, M.; D'Anna, R.; di Pasquale, G.; Floccari, F.; Ruello, A.; Aloisi, C.; Leonardi, I.; Frisina, N.; Corica, F. Urinary excretion of aquaporin-2 water channel during pregnancy. Cell. Physiol. Biochem. 2001, 11, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ivarsen, P.; Frokiaer, J.; Aagaard, N.K.; Hansen, E.F.; Bendtsen, F.; Nielsen, S.; Vilstrup, H. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Gut 2003, 52, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Funayama, H.; Nakamura, T.; Saito, T.; Yoshimura, A.; Saito, M.; Kawakami, M.; Ishikawa, S.E. Urinary excretion of aquaporin-2 water channel exaggerated dependent upon vasopressin in congestive heart failure. Kidney Int. 2004, 66, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.Y.; Abraham, W.T.; Lieming, X.; Olson, B.R.; Oren, R.M.; Ohara, M.; Schrier, R.W. Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J. Am. Soc. Nephrol. 1999, 10, 2165–2170. [Google Scholar] [PubMed]
- Imamura, T.; Kinugawa, K.; Hatano, M.; Fujino, T.; Inaba, T.; Maki, H.; Kinoshita, O.; Nawata, K.; Kyo, S.; Ono, M.; et al. Low cardiac output stimulates vasopressin release in patients with stage D heart failure. Circ. J. 2014, 78, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Francis, G.S.; Cowley, A.W., Jr.; Levine, T.B.; Cohn, J.N. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J. Am. Coll. Cardiol. 1983, 1, 1385–1390. [Google Scholar] [CrossRef]
- Szatalowicz, V.L.; Arnold, P.E.; Chaimovitz, C.; Bichet, D.; Berl, T.; Schrier, R.W. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N. Engl. J. Med. 1981, 305, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Pruszczynski, W.; Vahanian, A.; Ardaillou, R.; Acar, J. Role of antidiuretic hormone in impaired water excretion of patients with congestive heart failure. J. Clin. Endocrinol. Metab. 1984, 58, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Funayama, H.; Yoshimura, A.; Tsuruya, Y.; Saito, M.; Kawakami, M.; Ishikawa, S.E. Possible vascular role of increased plasma arginine vasopressin in congestive heart failure. Int. J. Cardiol. 2006, 106, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Bettari, L.; Fiuzat, M.; Shaw, L.K.; Wojdyla, D.M.; Metra, M.; Felker, G.M.; O'Connor, C.M. Hyponatremia and long-term outcomes in chronic heart failure—An observational study from the duke databank for cardiovascular diseases. J. Card. Fail. 2012, 18, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Haraguchi, G.; Ohigashi, H.; Sasaoka, T.; Yoshikawa, S.; Inagaki, H.; Ashikaga, T.; Isobe, M. Progression of hyponatremia is associated with increased cardiac mortality in patients hospitalized for acute decompensated heart failure. J. Card. Fail. 2012, 18, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Gheorghiade, M.; Kajimoto, K.; Munakata, R.; Minami, Y.; Mizuno, M.; Aokage, T.; Asai, K.; Sakata, Y.; Yumino, D.; et al. Hyponatremia and in-hospital mortality in patients admitted for heart failure (from the attend registry). Am. J. Cardiol. 2013, 111, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Abraham, W.T.; Albert, N.M.; Gattis Stough, W.; Greenberg, B.H.; O’Connor, C.M.; She, L.; Yancy, C.W.; Young, J.; Fonarow, G.C.; et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur. Heart J. 2007, 28, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Miura, K.; Iwao, H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J. Pharmacol. Sci. 2014, 124, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Minatsuki, S.; Muraoka, H.; Kato, N.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; Komuro, I. Tolvaptan can improve clinical course in responders. Int. Heart J. 2013, 54, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Shiga, T.; Kato, N.; Endo, M.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; Hirata, Y.; et al. Correction of hyponatremia by tolvaptan before left ventricular assist device implantation. Int. Heart J. 2012, 53, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Kato, N.; Minatsuki, S.; Muraoka, H.; Inaba, T.; Maki, H.; Shiga, T.; Hatano, M.; Yao, A.; et al. Successful conversion from thiazide to tolvaptan in a patient with stage D heart failure and chronic kidney disease before heart transplantation. Int. Heart J. 2013, 54, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, K.; Imamura, T.; Komuro, I. Experience of a vasopressin receptor antagonist, tolvaptan, under the unique indication in Japanese heart failure patients. Clin. Pharmacol. Ther. 2013, 94, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Costello-Boerrigter, L.C.; Smith, W.B.; Boerrigter, G.; Ouyang, J.; Zimmer, C.A.; Orlandi, C.; Burnett, J.C., Jr. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am. J. Physiol. Ren. Physiol. 2006, 290, F273–F278. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Gross, P.; Gheorghiade, M.; Berl, T.; Verbalis, J.G.; Czerwiec, F.S.; Orlandi, C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 2006, 355, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- Udelson, J.E.; Orlandi, C.; Ouyang, J.; Krasa, H.; Zimmer, C.A.; Frivold, G.; Haught, W.H.; Meymandi, S.; Macarie, C.; Raef, D.; et al. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: An international, multicenter, randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 2008, 52, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Berl, T.; Quittnat-Pelletier, F.; Verbalis, J.G.; Schrier, R.W.; Bichet, D.G.; Ouyang, J.; Czerwiec, F.S.; Investigators, S. Oral tolvaptan is safe and effective in chronic hyponatremia. J. Am. Soc. Nephrol. 2010, 21, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, K.; Sato, N.; Inomata, T.; Shimakawa, T.; Iwatake, N.; Mizuguchi, K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ. J. 2014, 78, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Hori, M.; Izumi, T.; Fukunami, M.; Tolvaptan, I. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: A phase III, randomized, double-blind, placebo-controlled study (quest study). Cardiovasc. Drugs Ther. 2011, 25, S33–S45. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K. Mid-term administration of tolvaptan improves renal function accompanied by dose-reduction in furosemide in aquaporin-defined responders. Int. Heart J. 2015, 56, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Kato, N.; Minatsuki, S.; Muraoka, H.; Inaba, T.; Maki, H.; Shiga, T.; Hatano, M.; Hosoya, Y.; et al. A case with recovery of response to tolvaptan associated with remission of acute kidney injury and increased urine osmolality. Int. Heart J. 2013, 54, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; Komuro, I. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ. J. 2014, 78, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The everest outcome trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, P.J.; Burnett, J.; Gheorghiade, M.; Grinfeld, L.; Konstam, M.A.; Kostic, D.; Krasa, H.B.; Maggioni, A.; Ouyang, J.; Swedberg, K.; et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J. Card. Fail. 2013, 19, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Shiga, T.; Kato, N.; Muraoka, H.; Minatsuki, S.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients—Association between non-responders and chronic kidney disease. Circ. J. 2013, 77, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Minatsuki, S.; Muraoka, H.; Kato, N.; Inaba, T.; Maki, H.; Shiga, T.; Hatano, M.; Yao, A.; et al. Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circ. J. 2013, 77, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Nakamura, K.; Nakahama, M.; Wada, T.; Watanabe, A.; Hashimoto, K.; Terasaka, R.; Tokioka, K.; Nishii, N.; Miyoshi, T.; et al. Clinical characteristics of responders to treatment with tolvaptan in patients with acute decompensated heart failure: Importance of preserved kidney size. J. Cardiol. 2015, 67, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Katsuno, T.; Ozaki, T.; Sakata, F.; Kato, N.; Suzuki, Y.; Kosugi, T.; Kato, S.; Tsuboi, N.; Sato, W.; et al. The efficacy of tolvaptan as a diuretic for chronic kidney disease patients. Acta Cardiol. 2015, 70, 217–223. [Google Scholar] [PubMed]
- Tominaga, N.; Kida, K.; Matsumoto, N.; Akashi, Y.J.; Miyake, F.; Kimura, K.; Shibagaki, Y. Safety of add-on tolvaptan in patients with furosemide-resistant congestive heart failure complicated by advanced chronic kidney disease: A sub-analysis of a pharmacokinetics/pharmacodynamics study. Clin. Nephrol. 2015, 84, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, H.; Kawabata, H.; Sakaguchi, Y.; Yamamoto, R.; Hamano, T.; Rakugi, H.; Isaka, Y. Urine osmolarity predicts the body weight-reduction response to tolvaptan in chronic kidney disease patients: A retrospective, observational study. Nephron 2015, 130, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T. Aquaporin-2-guided tolvaptan therapy in patients with congestive heart failure accompanied by chronic kidney disease. Int. Heart J. 2014, 55, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Mose, F.H.; Kulik, A.E.; Bech, J.N.; Fenton, R.A.; Pedersen, E.B. Abnormal urinary excretion of NKCC2 and AQP2 in response to hypertonic saline in chronic kidney disease: An intervention study in patients with chronic kidney disease and healthy controls. BMC Nephrol. 2014, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Nakamura, T.; Amaha, M.; Nomura, M.; Matsumura, D.; Yamagishi, H.; Ono, Y.; Ueda, Y. Effect of tolvaptan in patients with chronic kidney disease due to diabetic nephropathy with heart failure. Int. Heart J. 2014, 55, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Kakegawa, D.; Yamada, A.; Ito, A.; Miwa, S.; Ida, H. Tolvaptan in a pediatric patient with diuretic-resistant heart and kidney failure. Pediatr. Int. 2015, 57, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Sakai, Y.; Ohno, D.; Murasawa, T.; Sato, N.; Tsuruoka, S. The effects of tolvaptan on patients with severe chronic kidney disease complicated by congestive heart failure. Clin. Exp. Nephrol. 2013, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Kinugawa, K.; Komuro, I. Tolvaptan prolongs blockage of the vasopressin type II receptor over 24 hours in responders with stage D heart failure. Int. Heart J. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Kinugawa, K. Urine Aquaporin-2: A Promising Marker of Response to the Arginine Vasopressin Type-2 Antagonist, Tolvaptan in Patients with Congestive Heart Failure. Int. J. Mol. Sci. 2016, 17, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010105
Imamura T, Kinugawa K. Urine Aquaporin-2: A Promising Marker of Response to the Arginine Vasopressin Type-2 Antagonist, Tolvaptan in Patients with Congestive Heart Failure. International Journal of Molecular Sciences. 2016; 17(1):105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010105
Chicago/Turabian StyleImamura, Teruhiko, and Koichiro Kinugawa. 2016. "Urine Aquaporin-2: A Promising Marker of Response to the Arginine Vasopressin Type-2 Antagonist, Tolvaptan in Patients with Congestive Heart Failure" International Journal of Molecular Sciences 17, no. 1: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010105