Column Selection for Biomedical Analysis Supported by Column Classification Based on Four Test Parameters
Abstract
:1. Introduction
2. Results and Discussion
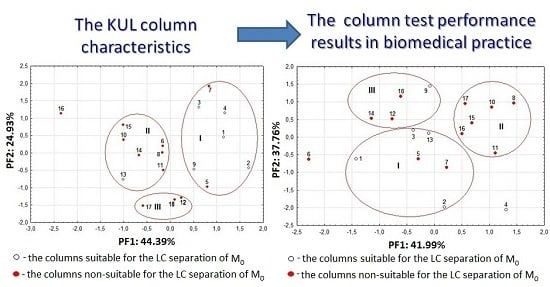
2.1. Column Classification
| Analytical Column | Column Parameters | FKUL | The Position in the Ranking List (Column No.) | |||
|---|---|---|---|---|---|---|
| k′amb | rk′tri/o-ter | rk′ba/ph pH2.7 | k′2,2′-d | |||
| Nuc_C18/125/5 | 5.09 | 1.57 | 0.116 | 21.07 | 0.00 | 1 |
| Nuc_C18/250/5 | 6.09 | 1.61 | 0.102 | 26.01 | 1.70 | 2 |
| SynPol_RP | 2.74 | 1.31 | 0.088 | 20.28 | 2.35 | 3 |
| Varian_C18 | 3.20 | 1.18 | 0.097 | 22.62 | 3.04 | 4 |
| NovPack_C18 | 9.42 | 1.39 | 0.108 | 18.21 | 3.97 | 5 |
| Nuc_C18/125/10 | 6.78 | 1.24 | 0.104 | 12.36 | 4.50 | 6 |
| Nuc_C8 | 1.08 | 1.75 | 0.125 | 19.35 | 4.73 | 7 |
| SynFus_RP | 6.54 | 1.05 | 0.050 | 17.61 | 4.74 | 8 |
| Luna_C18 (2) | 7.82 | 1.13 | 0.087 | 17.61 | 4.88 | 9 |
| Sym_C8 | 5.55 | 1.08 | 0.029 | 14.63 | 4.95 | 10 |
| Aqua_C18 | 8.18 | 1.24 | 0.074 | 15.34 | 5.07 | 11 |
| Inert_ODS2 | 9.67 | 1.68 | 0.072 | 19.25 | 5.94 | 12 |
| NucHD_C18 | 8.76 | 1.51 | 0.053 | 12.53 | 5.96 | 13 |
| GemNX_C18 | 7.19 | 1.12 | 0.061 | 13.12 | 5.97 | 14 |
| Inert_C8 | 4.61 | 1.02 | 0.040 | 13.40 | 6.22 | 15 |
| SymShield_C8 | 3.46 | 1.78 | 0.031 | 7.83 | 7.17 | 16 |
| Sym_C18 | 10.57 | 1.53 | 0.049 | 15.62 | 9.04 | 17 |
| SynMax_RP | 10.04 | 1.15 | 0.046 | 19.43 | 9.12 | 18 |

2.2. Column Selectivity in Analysis of Moclobemide
| Substances: | M0 | M1 | M2 | Phenacetin (I.S.) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Analytical Column: | tR | tR | Rs | tR | Rs | tR | Rs |
| 1 | Nuc_C18/125/5 | 2.51 | 2.95 | 1.61 | 5.15 | 8.39 | 5.53 | 1.64 |
| 2 | Nuc_C18/250/5 | 3.95 | 4.56 | 2.56 | 7.31 | 8.44 | 8.75 | 3.25 |
| 3 | SynPol_RP | 3.07 | 3.46 | 2.74 | 7.40 | 14.81 | 8.43 | 3.31 |
| 4 | Varian_C18 | 7.95 | 9.35 | 1.82 | 10.33 | 1.58 | 11.95 | 2.02 |
| 5 | NovPack_C18 | 3.46 | 4.05 | 1.71 | 7.16 | 5.42 | 7.83 | 0.83 |
| 6 | Nuc_C18/125/10 | 1.50 | 1.63 | 1.13 | 3.08 | 4.83 | 3.08 | 0.00 |
| 7 | Nuc_C8 | 3.96 | 4.81 | 1.97 | 11.08 | 8.48 | 11.08 | 0.00 |
| 8 | SynFus_RP | 4.03 | 4.03 | 0.00 | 14.13 | 31.43 | 16.53 | 3.98 |
| 9 | Luna_C18 (2) | 1.61 | 2.28 | 5.54 | 8.15 | 26.91 | 9.56 | 4.09 |
| 10 | Sym_C8 | 3.80 | 3.80 | 0.00 | 12.90 | 24.42 | 15.03 | 4.96 |
| 11 | Aqua_C18 | 4.55 | 5.15 | 0.78 | 13.61 | 11.30 | 15.41 | 1.91 |
| 12 | Inert_ODS2 | 2.20 | 2.20 | 0.00 | 7.43 | 18.84 | 8.76 | 3.32 |
| 13 | NucHD_C18 | 3.45 | 3.95 | 2.60 | 8.13 | 14.24 | 9.48 | 3.41 |
| 14 | GemNX_C18 | 2.06 | 2.06 | 0.00 | 5.73 | 17.20 | 7.00 | 4.03 |
| 15 | Inert_C8 | 3.90 | 3.90 | 0.00 | 11.71 | 18.86 | 13.68 | 4.24 |
| 16 | SymShield_C8 | 3.75 | 3.75 | 0.00 | 11.16 | 14.13 | 14.23 | 3.27 |
| 17 | Sym_C18 | 3.33 | 3.33 | 0.00 | 11.10 | 29.63 | 13.06 | 4.05 |
| 18 | SynMax_RP | 2.21 | 2.21 | 0.00 | 6.83 | 23.68 | 8.73 | 5.89 |

3. Experimental Section
3.1. Column Examination
3.2. Chemicals
3.3. Equipment and LC Conditions
3.4. Column Classification
3.5. Column Test Performance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Euerby, M.R.; Petterson, P. A classification of commercially available RPLC columns—A tool for rational selection. LC-GC Eur. 2000, 13, 665–677. [Google Scholar]
- Jandera, P.; Novotná, K. Characterization of high-pressure liquid chromatography columns using chromatographic methods. Anal. Lett. 2006, 39, 2095–2152. [Google Scholar] [CrossRef]
- Forlay-Frick, P.; Fekete, J.; Héberger, K. Classification and replacement test of HPLC systems using principal component analysis. Anal. Chim. Acta 2005, 536, 71–81. [Google Scholar] [CrossRef]
- Lesellier, E.; West, C. Description and comparison of chromatographic tests and chemometric methods for packed column classification. J. Chromatogr. A 2007, 1158, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Galushko, S.V.; Kamenchuk, A.A.; Pit, G.L. Calculation of retention in reversed-phase liquid chromatography: IV. ChromDream software for the selection of initial conditions and for simulating chromatographic behaviour. J. Chromatogr. A 1994, 660, 47–59. [Google Scholar] [CrossRef]
- Stella, C.; Rudaz, S.; Veuthey, J.-L.; Tchapla, A. Silica and other materials as supports in liquid chromatography. Chromatographic tests and their importance for evaluating these supports. Part I. Chromatographia 2001, 53 (Suppl. 1), S113–S131. [Google Scholar] [CrossRef]
- Stella, C.; Rudaz, S.; Veuthey, J.-L.; Tchapla, A. Silica and other materials as supports in liquid chromatography. Chromatographic tests and their importance for evaluating these supports. Part II. Chromatographia 2001, 53 (Suppl. 1), S132–S140. [Google Scholar] [CrossRef]
- Kaliszan, R.; van Straten, M.A.; Markuszewski, M.; Cramers, C.A.; Claessens, H.A. Molecular mechanism of retention in reversed-phase high-performance liquid chromatography and classification of modern stationary phases by using quantitative structure–retention relationships. J. Chromatogr. A 1999, 855, 455–486. [Google Scholar] [CrossRef]
- Bączek, T.; Kaliszan, R.; Novotńa, K.; Jandera, P. Comparative characteristics of HPLC columns based on quantitative structure-retention relationships (QSRR) and hydrophobic-subtraction model. J. Chromatogr. A 2005, 1075, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kaliszan, R. QSRR: Quatitative Structure-(Chromatographic) Retention Relationships. Chem. Rev. 2007, 107, 3212–3246. [Google Scholar] [CrossRef] [PubMed]
- Plenis, A.; Konieczna, L.; Miękus, N.; Bączek, T. Development of the HPLC method for simultaneous determination of lidocaine hydrochloride and tribenoside along with their impurities supported by the QSRR approach. Chromatography 2013, 76, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.R.; Dolan, J.W.; Carr, P.W. The hydrophobic-subtraction model of reversed-phase column selectivity. J. Chromatogr. A 2004, 1060, 77–116. [Google Scholar] [CrossRef]
- Snyder, L.R.; Dolan, J.W.; Carr, P.W. A new look at the selectivity of RPC columns. Anal. Chem. 2007, 79, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Kimata, K.; Iwaguchi, K.; Onishi, S.; Jinno, K.; Eksteen, R.; Hosoya, K.; Arki, M.; Tanaka, N. Chromatographic Characterization of Silica C18 Packing Materials. Correlation between a Preparation Method and Retention Behavior of Stationary Phase. J. Chromatogr. Sci. 1989, 27, 721–728. [Google Scholar] [CrossRef]
- Euerby, M.R.; Petersson, P. Chromatographic classification and comparison of commercially available reversed-phase liquid chromatographic columns containing polar embedded groups/amino endcappings using principal component analysis. J. Chromatogr. A 2005, 1088, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, C.; Tweedlie, T.; Watson, D.; Skellern, G.G.; Reda, H.; Petersson, P.; Bradstock, H.; Euerby, M.R. A Study of the Relative Importance of Lipophilic, π-π and Dipole-Dipole Interactions on Cyanopropyl, Phenyl and Alkyl LC Phases Bonded onto the Same Base Silica. Chromatographia 2009, 70, 705–715. [Google Scholar] [CrossRef]
- Euerby, M.R.; James, M.; Petersson, P. Practical implications of the “Tanaka” stationary phase characterization methodology using ultra high performance liquid chromatographic conditions. J. Chromatogr. A 2012, 1228, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.; Euerby, M.R.; Johnson, C.M.; Hackett, C.A. Chromatographic classification of commercially available reversed-phase HPLC columns. Chromatographia 1997, 44, 151–161. [Google Scholar] [CrossRef]
- Visky, D.; Heyden, Y.V.; Iványi, T.; Baten, P.; de Beer, J.; Kovács, Z.; Noszál, B.; Roets, E.; Massart, D.L.; Hoogmartens, J. Characterisation of reversed-phase liquid chromatographic columns by chromatographic tests. Evaluation of 36 test parameters: Repeatability, reproducibility and correlation. J. Chromatogr. A 2002, 977, 39–58. [Google Scholar] [CrossRef]
- Stella, C.; Rudaz, S.; Gauvrit, J.-Y.; Lanteri, P.; Huteau, A.; Tchapla, A.; Veuthey, J.L. Characterization and comparison of the chromatographic performance of different types of reversed-phase stationary phases. J. Pharm. Biomed. Anal. 2007, 43, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Stella, C.; Seuret, P.; Rudaz, S.; Tchapla, A.; Gauvrit, J.Y.; Lanteri, P.; Veuthey, J.L. Simplification of a chromatographic test methodology for evaluation of base deactivated supports. Chromatographia 2002, 56, 665–671. [Google Scholar] [CrossRef]
- Fekete, S.; Veuthey, J.L.; Guillarme, D. Comparison of the most recent chromatographic approaches applied for fast and high resolution separations: Theory and practice. J. Chromatogr. A 2015, 1408, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, H.; Jungheim, M. Comparison and characterization of reversed phases. Chromatographia 1990, 29, 59–68. [Google Scholar] [CrossRef]
- Claessens, H.A.; van Straten, M.A.; Cramers, C.A.; Jezierska, M.; Buszewski, B. Comparative study of test methods for reversed-phase columns for high-performance liquid chromatography. J. Chromatogr. A 1998, 826, 135–156. [Google Scholar] [CrossRef]
- Van Gyseghem, E.; Jimidar, M.; Sneyers, R.; de Smet, M.; Verhoeven, E.; Vander Heyden, Y. Stationary phases in the screening of drug/impurity profiles and in their separation method development: Identification of columns with different and similar selectivities. J. Pharm. Biomed. Anal. 2006, 41, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bonose-Crosnier de Bellaistre, M.; Nowik, W.; Tchapla, A.; Heron, S. Separation of 9,10-anthraquinone derivatives: Evaluation of C18 stationary phases. J. Chromatogr. A 2011, 1218, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Héberger, K. Sum of ranking differences compares methods or models fairly. Trends Anal. Chem. 2010, 29, 101–109. [Google Scholar] [CrossRef]
- Dehouck, P.; Visky, D.; Vander Heyden, Y.; Adams, E.; Kovács, Z.; Noszál, B.; Massart, D.L.; Hoogmartens, J. Characterization of reversed-phase liquid-chromatographic columns by chromatographic tests. Comparing column classification based on chromatographic parameters and column performance for the separation of acetylsalicylic acid and related compounds. J. Chromatogr. A 2004, 1025, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, P.; Visky, D.; van den Bergh, G.; Haghedooren, E.; Adams, E.; Kerner, A.; Vander Heyden, Y.; Massart, D.L.; Kovács, Z.; Noszál, B.; et al. Facilitated column selection in reversed-phase liquid chromatography for pharmaceutical separations. LC-GC Eur. 2004, 17, 592–601. [Google Scholar]
- Haghedooren, E.; Kerner, A.; Noszál, B.; Hoogmartens, J.; Adams, E. Application of an improved column characterization system to evaluate the within and between batch variability. J. Pharm. Biomed. Anal. 2007, 44, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Visky, D.; Haghedooren, E.; Dehouck, P.; Kovács, Z.; Kóczián, K.; Noszál, B.; Hoogmartens, J.; Adams, E. Facilitated column selection in pharmaceutical analyses using a simple column classification system. J. Chromatogr. A 2006, 1101, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Haghedooren, E.; Németh, T.; Dragovic, S.; Noszál, B.; Hoogmartens, J.; Adams, E. Comparison of two column characterization systems based on pharmaceutical applications. J. Chromatogr. A 2008, 1189, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Haghedooren, E.; Diana, J.; Noszál, B.; Hoogmartens, J.; Adams, E. Classification of reversed phase columns based on their selectivity towards vancomycin compounds. Talanta 2007, 71, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kóczián, K.; Haghedooren, E.; Dragovic, S.; Noszál, B.; Hoogmartens, J.; Adams, E. Column selection for pharmaceutical analyses based on a column classification using four test parameters. J. Pharm. Biomed. Anal. 2007, 44, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Szulfer, J.; Plenis, A.; Bączek, T. Comparison of core-shell and totally porous ultra high performance liquid chromatographic stationary phases based on their selectivity towards alfuzosin compounds. J. Chromatogr. A 2014, 1346, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Németh, T.; Haghedooren, E.; Noszál, B.; Hoogmartens, J.; Adams, E. Three methods to characterize reversed phase liquid chromatographic columns applied to pharmaceutical separations. J. Chemometr. 2008, 22, 178–185. [Google Scholar] [CrossRef]
- Dragovic, S.; Haghedooren, E.; Nemeth, T.; Palabiyik, I.M.; Hoogmartens, J.; Adams, E. Evaluation of two approaches to characterise liquid chromatographic columns using pharmaceutical separations. J. Chromatogr. A 2009, 1216, 3210–3216. [Google Scholar] [CrossRef] [PubMed]
- Plenis, A.; Balakowska, E.; Bączek, T. The comparison of two column classification systems during the chromatographic analysis of steroids. J. Sep. Sci. 2011, 34, 3310–3321. [Google Scholar] [CrossRef] [PubMed]
- Szulfer, J.; Plenis, A.; Bączek, T. Evaluation of a column classification method using the separation of alfuzosin from its related substances. J. Chromatogr. A 2012, 1229, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Szulfer, J.; Plenis, A.; Bączek, T. Application of a column classification method in a selectivity study involving caffeine and its related impurities. Talanta 2012, 99, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Szulfer, J.; Plenis, A.; Bączek, T. Chemometric evaluation of the column classification system during the pharmaceutical analysis of lamotrigine and its related substances. Anal. Bioanal. Chem. 2013, 405, 6529–6541. [Google Scholar] [CrossRef] [PubMed]
- Plenis, A.; Chmielewska, A.; Konieczna, L.; Lamparczyk, H. A validated high-performance liquid chromatographic method for the determination of moclobemide and its two metabolites in human plasma and application to pharmacokinetic studies. Biomed. Chromatogr. 2007, 21, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Plenis, A.; Olędzka, I.; Bączek, T. Classification of LC columns based on the QSRR method and selectivity toward moclobemide and its metabolites. J. Pharm. Biomed. Anal. 2013, 78–79, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Forina, M.; Armanino, C.; Lanteri, S.; Leardi, R. Methods of varimax rotation in factor analysis with applications in clinical and food chemistry. J. Chemometr. 1989, 3, 115–125. [Google Scholar] [CrossRef]
- Hao, Z.; Xiao, B.; Weng, N. Impact of column temperature and mobile phase components on selectivity of hydrophilic interaction chromatography (HILIC). J. Sep. Sci. 2008, 31, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Pataj, Z.; Gross, H.; Sardella, R.; Natalini, B.; Lindner, W.; Lämmerhofer, M. Direct enantioseparation of underivatized aliphatic 3-hydroxyalkanoic acids with a quinine-based zwitterionic chiral stationary phase. J. Chromatogr. A 2014, 1363, 101–108. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plenis, A.; Rekowska, N.; Bączek, T. Column Selection for Biomedical Analysis Supported by Column Classification Based on Four Test Parameters. Int. J. Mol. Sci. 2016, 17, 136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010136
Plenis A, Rekowska N, Bączek T. Column Selection for Biomedical Analysis Supported by Column Classification Based on Four Test Parameters. International Journal of Molecular Sciences. 2016; 17(1):136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010136
Chicago/Turabian StylePlenis, Alina, Natalia Rekowska, and Tomasz Bączek. 2016. "Column Selection for Biomedical Analysis Supported by Column Classification Based on Four Test Parameters" International Journal of Molecular Sciences 17, no. 1: 136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010136