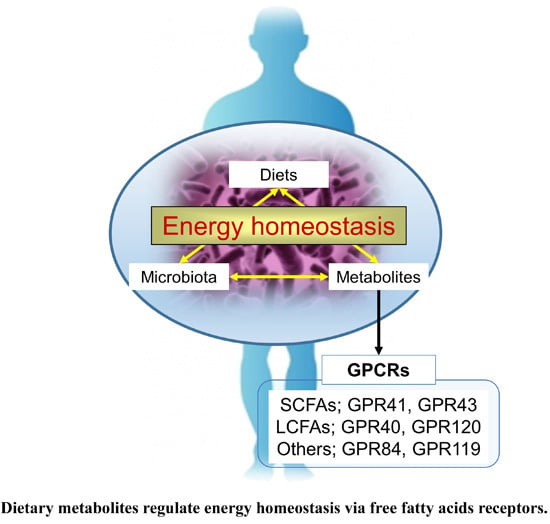
Nutritional Signaling via Free Fatty Acid Receptors
Abstract
:1. Introduction
2. GPR40/FFAR1
3. GPR120/FFAR4
4. GPR41/FFAR3
5. GPR43/FFAR2
6. Other GPCRs
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Santacruz, A.; Gauffin, P. Gut microbiota in obesity and metabolic disorders. Proc. Nutr. Soc. 2010, 69, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.C.; Bloom, S.R.; Murphy, K.G. Peptides and their potential role in the treatment of diabetes and obesity. Rev. Diabet. Stud. 2011, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.; Bäckhed, F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011, 22, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Nieuwdorp, M.; Bäckhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 2014, 20, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. A place for dietary fibre in the management of the metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Endocrinol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G. Gut microbiome, obesity-related comorbidities, and low-grade chronic inflammation. J. Clin. Endocrinol. Metab. 2014, 99, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Harig, J.M.; Soergel, K.H.; Komorowski, R.A.; Wood, C.M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N. Engl. J. Med. 1989, 320, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, K.; Nilsson, N.E.; Flodgren, E.; Owman, C.; Olde, B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem. Biophys. Res. Commun. 2003, 301, 406–410. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Hara, T.; Kimura, I.; Inoue, D.; Ichimura, A.; Hirasawa, A. Free fatty acid receptors and their role in regulation of energy metabolism. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 77–116. [Google Scholar] [PubMed]
- Welters, H.J.; Diakogiannaki, E.; Mordue, J.M.; Tadayyon, M.; Smith, S.A.; Morgan, N.G. Differential protective effects of palmitoleic acid and cAMP on caspase activation and cell viability in pancreatic β-cells exposed to palmitate. Apoptosis 2006, 11, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Steneberg, P.; Rubins, N.; Bartoov-Shifman, R.; Walker, M.D.; Edlund, H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005, 1, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Nagasumi, K.; Esaki, R.; Iwachidow, K.; Yasuhara, Y.; Ogi, K.; Tanaka, H.; Nakata, M.; Yano, T.; Shimakawa, K.; Taketomi, S.; et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 2009, 58, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.; Smith, D.M.; Bergsten, P.; Sargsyan, E. FFAR1 is involved in both the acute and chronic effects of palmitate on insulin secretion. Endocrinology 2013, 154, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Sykaras, A.G.; Demenis, C.; Case, R.M.; McLaughlin, J.T.; Smith, C.P. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE 2012, 7, e42373. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 407–434. [Google Scholar] [CrossRef] [PubMed]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Hara, T.; Katsuma, S.; Adachi, T.; Tsujimoto, G. Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 2008, 31, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, C.; Yasumatsu, K.; Ohkuri, T.; Shigemura, N.; Yoshida, R.; Godinot, N.; le Coutre, J.; Ninomiya, Y.; Damak, S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010, 30, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.P.; Lu, X.; Sei, Y.; Zhao, X.; Pechhold, S.; Carrero, R.J.; Raybould, H.E.; Wank, S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011, 140, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Tao, B.; Warashina, S.; Kotani, S.; Lu, L.; Kaplamadzhiev, D.B.; Mori, Y.; Tonchev, A.B.; Yamashima, T. Expression of free fatty acid receptor GPR40 in the central nervous system of adult monkeys. Neurosci. Res. 2007, 58, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Ichimura, A.; Hirasawa, A. Therapeutic role and ligands of medium- to long-chain fatty acid receptors. Front. Endocrinol. Lausanne 2014, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Hosoda, K.; Fujikura, J.; Inagaki, N.; Nakao, K. The G-Protein-Coupled Long-Chain Fatty Acid Receptor GPR40 and Glucose Metabolism. Front. Endocrinol. Lausanne 2014, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Mizukure, T.; Park, S.B.; Kishino, S.; Kimura, I.; Hirano, K.; Bergamo, P.; Rossi, M.; Suzuki, T.; Arita, M.; et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 2015, 290, 2902–2918. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.A.; Nahuelpan, C.; Manosalva, C.; Jara, E.; Carretta, M.D.; Conejeros, I.; Loaiza, A.; Chihuailaf, R.; Burgos, R.A. Oleic acid induces intracellular calcium mobilization, MAPK phosphorylation, superoxide production and granule release in bovine neutrophils. Biochem. Biophys. Res. Commun. 2011, 409, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, T.; Kurata, R.; Yoshida, K.; Murayama, M.A.; Cui, X.; Hasegawa, A. Free fatty acids-sensing G protein-coupled receptors in drug targeting and therapeutics. Curr. Med. Chem. 2013, 20, 3855–3871. [Google Scholar] [CrossRef] [PubMed]
- Zamarbide, M.; Etayo-Labiano, I.; Ricobaraza, A.; Martínez-Pinilla, E.; Aymerich, M.S.; Lanciego, L.J.; Pérez-Mediavilla, A.; Franco, R. GPR40 activation leads to CREB and ERK phosphorylation in primary cultures of neurons from the mouse CNS and in human neuroblastoma cells. Hippocampus 2014, 24, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Yano, J.; Hirozane, Y.; Kefala, G.; Gruswitz, F.; Snell, G.; Lane, W.; Ivetac, A.; Aertgeerts, K.; Nguyen, J.; et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature 2014, 513, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Hirasawa, A.; Sun, Q.; Sadakane, K.; Itsubo, C.; Iga, T.; Adachi, T.; Koshimizu, T.A.; Hashimoto, T.; Asakawa, Y.; et al. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Katsuma, S.; Hatae, N.; Yano, T.; Ruike, Y.; Kimura, M.; Hirasawa, A.; Tsujimoto, G. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J. Biol. Chem. 2005, 280, 19507–19515. [Google Scholar] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Thompson, D.G.; Warhurst, G.; Case, R.M.; Benson, R.S. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J. Physiol. 2000, 528, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Katsuma, S.; Adachi, T.; Koshimizu, T.A.; Hirasawa, A.; Tsujimoto, G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, C.; Hong, Y.H.; Iga, T.; Hishikawa, D.; Suzuki, Y.; Song, S.H.; Choi, K.C.; Adachi, T.; Hirasawa, A.; Tsujimoto, G.; et al. The regulation of adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 2007, 354, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar]
- Tazoe, H.; Otomo, Y.; Karaki, S.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Gröne, H.J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Darzi, J.; Frost, G.S.; Robertson, M.D. Do SCFA have a role in appetite regulation? Proc. Nutr. Soc. 2011, 70, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.D.; Due-Hansen, M.E.; Christiansen, E.; Hansen, A.M.; Mackenzie, A.E.; Murdoch, H.; Pandey, S.K.; Ward, R.J.; Marquez, R.; Tikhonova, I.G.; et al. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J. Biol. Chem. 2013, 288, 17296–17312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.C.; Feng, D.D.; Chen, C.; Lee, H.G.; et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA Receptor GPR43 and Energy Metabolism. Front. Endocrinol. Lausanne 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, A. Contributions of colonic short-chain fatty acid receptors in energy homeostasis. Front. Endocrinol. Lausanne 2014, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Sina, C.; Gavrilova, O.; Förster, M.; Till, A.; Derer, S.; Hildebrand, F.; Raabe, B.; Chalaris, A.; Scheller, J.; Rehmann, A.; et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009, 183, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Overton, H.A.; Babbs, A.J.; Doel, S.M.; Fyfe, M.C.; Gardner, L.S.; Griffin, G.; Jackson, H.C.; Procter, M.J.; Rasamison, C.M.; Tang-Christensen, M.; et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Vilsbøll, T.; Bagger, J.I.; Holst, J.J.; Knop, F.K. Increased postprandial GIP and glucagon responses, but unaltered GLP-1 response after intervention with steroid hormone, relative physical inactivity, and high-calorie diet in healthy subjects. J. Clin. Endocrinol. Metab. 2011, 96, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Odori, S.; Hosoda, K.; Tomita, T.; Fujikura, J.; Kusakabe, T.; Kawaguchi, Y.; Doi, R.; Takaori, K.; Ebihara, K.; Sakai, Y.; et al. GPR119 expression in normal human tissues and islet cell tumors: Evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism 2013, 62, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.L.; Carroll, C.; Chen, R.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Xing, C.; Sebring, K.; Zhou, J.; et al. N-Oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol. Endocrinol. 2010, 24, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.L.; Jones, R.M.; He, H.; Carroll, C.; Gutierrez, V.; Lucman, A.; Moloney, M.; Gao, H.; Mondala, H.; Bagnol, D.; et al. A role for β-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 2007, 148, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Lin, H.V.; Wang, C.F.; Wright, M.J.; Xu, S.; Kang, L.; Juhl, K.; Hedrick, J.A.; Kowalski, T.J. Agonists at GPR119 mediate secretion of GLP-1 from mouse enteroendocrine cells through glucose-independent pathways. Br. J. Pharmacol. 2012, 165, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Simonavicius, N.; Tian, H.; Ling, L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J. Biol. Chem. 2006, 281, 34457–34464. [Google Scholar] [CrossRef] [PubMed]
- Lattin, J.E.; Schroder, K.; Su, A.I.; Walker, J.R.; Zhang, J.; Wiltshire, T.; Saijo, K.; Glass, C.K.; Hume, D.A.; Kellie, S.; et al. Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res. 2008, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, C.; Kuo, F. The G-protein coupled receptor, GPR84 regulates IL-4 production by T lymphocytes in response to CD3 crosslinking. Immunol. Lett. 2005, 101, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, H.; Kondo, T.; Fuchigami, M.; Hashimoto, H.; Sugimura, Y.; Ozaki, N.; Arima, H.; Ota, A.; Oiso, Y.; Hamada, Y. Inflammatory changes in adipose tissue enhance expression of GPR84, a medium-chain fatty acid receptor: TNFα enhances GPR84 expression in adipocytes. FEBS Lett. 2012, 586, 368–372. [Google Scholar] [CrossRef] [PubMed]

| Ligand | EC50 of Ligand Affinity (μM) | |||
|---|---|---|---|---|
| GPR41/FFAR3 | GPR43/FFAR2 | GPR40/FFAR1 | GPR120/FFAR4 | |
| Saturated fatty acids | ||||
| acetic acid (C2:0) | >1000 (b–d) | 35–431 (b–d) | ||
| propionic acid (C3:0) | 6–127 (b–d) | 14–290 (b–d) | ||
| butyric acid (C4:0) | 42–158 (b–d) | 28–371 (b–d) | ||
| valeric acid (C5:0) | 42–142 (b–d) | >1000 (b–d) | ||
| caproic acid (C6:0) | 102–134 (a,c,d) | 46 (a,c,d) | ||
| caprylate (C8:0) | 38 (a) | |||
| capric acid (C10:0) | 14–43 (a,d) | |||
| lauric acid (C12:0) | 6–12 (a,d) | |||
| myristic acid (C14:0) | 8–14 (a,d) | 30 (a,d) | ||
| palmitic acid (C16:0) | 5–7 (a,d) | 52 (a,d) | ||
| stearic acid (C18:0) | 17 (a) | 18 (a) | ||
| Monounsaturated fatty acids | ||||
| palmitoleic acid (C16:1, n-7) | 14 (a) | 0.7–3 (a) | ||
| oleic acid (C18:1, n-9) | 2–40 (a,d) | 31 (a,d) | ||
| ω-3 fatty acids | ||||
| α-linolenic acid (C18:3, n-3) | 2–13 (a,d) | 0.5 (a,d) | ||
| cis-11,14,17-eicosatrienoic acid (C20:3, n-3) | 11 (a) | 1 (a) | ||
| cis-5,8,11,14,17-eicosapentaenoic acid (C20:5, n-3) | 2–7 (a,d) | 2–3 (a,d) | ||
| docosahexaenoic acid (22:6, n-3) | 1–4 (a,d) | 4 (a,d) | ||
| ω -6 fatty acids | ||||
| linoleic acid (C18:2, n-6) | 2–10 (a,d) | 1 (a,d) | ||
| γ-linolenic acid (C18:3, n-6) | 5–9 (a,d) | 1 (a,d) | ||
| dihomo-γ-linolenic acid (C20:4, n-6) | 7 (a) | 14 (a) | ||
| arachidonic acid (C20:4, n-6) | 2–12 (a,d) | |||
| docosatetraenoic acid (C22:4, n-6) | 13 (a) | 16 (a) | ||
| Ligand | EC50 (μM) |
|---|---|
| all trans-retinoic acid | no response |
| C18:1 LPC | no response |
| oleoylethanolamide (OEA) | 4.4 |
| Z-capsaicin | no response |
| olvanil | 7.8 |
| N-oleoyldopamine (OLDA) | 3.2 |
| (R)-N-oleoyltyrosinol | 0.5 |
| (S)-N-oleoyltyrosinol | 0.7 |
| N-oleoyltryosine | no response |
| N-arachidonyldopamine (NADA) | no response |
| Ligand | EC50 (μM) |
|---|---|
| formic acid (C1:0) | no response |
| acetic acid (C2:0) | no response |
| propionic acid (C3:0) | no response |
| butyric acid (C4:0) | no response |
| caproic acid (C6:0) | no response |
| heptanoic acid (C7:0) | no response |
| caprylic acid (C8:0) | no response |
| nonanoic acid (C9:0) | 52.3 ± 5.6 |
| capric acid (C10:0) | 4.5 ± 0.3 |
| undecanoic acid (C11:0) | 7.7 ± 0.1 |
| lauric acid (C12:0) | 8.8 ± 0.2 |
| tridecanoic acid (C13:0) | 24.8 ± 1.1 |
| myristic acid (C14:0) | 93.2 ± 11.0 |
| pentadeconoic acid (C15:0) | no response |
| palmitic acid (C16:0) | no response |
| heptadecanoic acid (C17:0) | no response |
| stearic acid (C18:0) | no response |
| arachidic acid (C20:0) | no response |
| heneicosanoic acid (C21:0) | no response |
| behenic acid (C22:0) | no response |
| palmitoleic acid (C16:1) | no response |
| oleic acid (C18:1) | no response |
| elaidic acid (C18:1) | no response |
| linoleic acid (C18:2) | no response |
| α-linolenic acid (C18:3) | no response |
| γ-linolenic acid (C18:3) | no response |
| cis-11,14,17-eicosatrienoic acid (C20:3) | no response |
| arachidonic acid (C20:4) | no response |
| cis-5,8,11,14,17-eicosapentaenoic acid (C20:5) | no response |
| docosahexaenoic acid (C22:6) | no response |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, J.; Hasegawa, S.; Kasubuchi, M.; Ichimura, A.; Nakajima, A.; Kimura, I. Nutritional Signaling via Free Fatty Acid Receptors. Int. J. Mol. Sci. 2016, 17, 450. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040450
Miyamoto J, Hasegawa S, Kasubuchi M, Ichimura A, Nakajima A, Kimura I. Nutritional Signaling via Free Fatty Acid Receptors. International Journal of Molecular Sciences. 2016; 17(4):450. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040450
Chicago/Turabian StyleMiyamoto, Junki, Sae Hasegawa, Mayu Kasubuchi, Atsuhiko Ichimura, Akira Nakajima, and Ikuo Kimura. 2016. "Nutritional Signaling via Free Fatty Acid Receptors" International Journal of Molecular Sciences 17, no. 4: 450. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17040450