The Prognostic and Predictive Role of Epidermal Growth Factor Receptor in Surgical Resected Pancreatic Cancer
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics According to EGFR (Epidermal Growth Factor Receptor) Expression
2.2. EGFR Status Was Not Correlated with Overall Survival after Surgical Resection
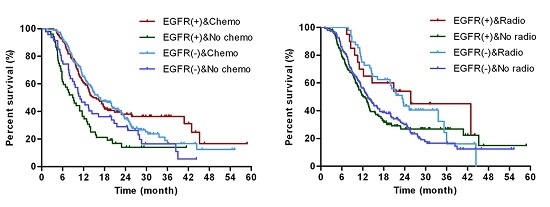
2.3. EGFR Status Was Not Correlated with Overall Survival after Surgical Resection Patients
2.4. EGFR Status Was Not Correlated with Overall Survival after Surgical Resection Patients
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Adjuvant Therapy
4.3. Tissue Samples
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moyer, M.T.; Gaffney, R.R. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Bukki, J. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2139–2140. [Google Scholar] [PubMed]
- Wolfgang, C.L.; Herman, J.M.; Laheru, D.A.; Klein, A.P.; Erdek, M.A.; Fishman, E.K.; Hruban, R.H. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013, 63, 318–348. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.; Berlin, J.; Cardin, D.B. Current treatment options for pancreatic carcinoma. Curr. Oncol. Rep. 2011, 13, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Ogata, S.; Tsuda, H.; Kawarabayashi, N.; Kimura, M.; Sugiura, Y.; Tamai, S.; Matsubara, O.; Hatsuse, K.; Mochizuki, H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: Poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 2004, 29, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Faller, B.A.; Burtness, B. Treatment of pancreatic cancer with epidermal growth factor receptor-targeted therapy. Biol. Targets Ther. 2009, 3, 419–428. [Google Scholar]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Cunningham, D.; Friess, H.; Bassi, C.; Stocken, D.D.; Tait, D.M.; Dunn, J.A.; Dervenis, C.; Lacaine, F.; Hickey, H.; et al. Adjuvant therapy in pancreatic cancer: Historical and current perspectives. Ann. Oncol. 2003, 14, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Bahra, M.; Schirmeier, A.; Al-Abadi, H.; Pratschke, J.; Pelzer, U.; Oettle, H.; Striefler, J.; Riess, H.; Sinn, M. Prognostic significance of DNA cytometry for adjuvant therapy response in pancreatic cancer. J. Surg. Oncol. 2015, 112, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ciardello, F.; Tortora, G. EGFR antagonists in cancer treatment (vol 358, pg 1160, 2008). N. Engl. J. Med. 2009, 360, 1579. [Google Scholar]
- Ciardiello, F.; Tortora, G. Drug therapy: EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Modjtahedi, H.; Dean, C. The receptor for EGF and its ligands—Expression, prognostic value and target for therapy in cancer (review). Int. J. Oncol. 1994, 4, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Friess, H.; Kleeff, J.; Korc, M.; Buchler, M.W. Molecular aspects of pancreatic cancer and future perspectives. Dig. Surg. 1999, 16, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Tobita, K.; Kijima, H.; Dowaki, S.; Kashiwagi, H.; Ohtani, Y.; Oida, Y.; Yamazaki, H.; Nakamura, M.; Ueyama, Y.; Tanaka, M.; et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int. J. Mol. Med. 2003, 11, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Ohuchida, K.; Mizumoto, K.; Itaba, S.; Ito, T.; Nakata, K.; Yu, J.; Kayashima, T.; Hayashi, A.; Souzaki, R.; et al. High EGFR mRNA expression is a prognostic factor for reduced survival in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Int. J. Oncol. 2011, 38, 629–641. [Google Scholar] [PubMed]
- Li, X.; Truty, M.A.; Kang, Y.A.; Chopin-Laly, X.; Zhang, R.; Roife, D.; Chatterjee, D.; Lin, E.; Thomas, R.M.; Wang, H.; et al. Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery. Clin. Cancer Res. 2014, 20, 6529–6540. [Google Scholar] [CrossRef] [PubMed]
- Kuniyasu, H.; Abbruzzese, J.L.; Cleary, K.R.; Fidler, I.J. Induction of ductal and stromal hyperplasia by basic fibroblast growth factor produced by human pancreatic carcinoma. Int. J. Oncol. 2001, 19, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Einama, T.; Ueda, S.; Tsuda, H.; Ogasawara, K.; Hatsuse, K.; Matsubara, O.; Todo, S.; Yamamoto, J. Membranous and cytoplasmic expression of epidermal growth factor receptor in metastatic pancreatic ductal adenocarcinoma. Exp. Ther. Med. 2012, 3, 931–936. [Google Scholar] [PubMed]
- Valsecchi, M.E.; McDonald, M.; Brody, J.R.; Hyslop, T.; Freydin, B.; Yeo, C.J.; Solomides, C.; Peiper, S.C.; Witkiewicz, A.K. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer 2012, 118, 3484–3493. [Google Scholar] [CrossRef] [PubMed]
- Bloomston, M.; Bhardwaj, A.; Ellison, E.C.; Frankel, W.L. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig. Surg. 2006, 23, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, H.G.; Erdmann, J.; van Dekken, H.; van Marion, R.; Hop, W.C.; Jeekel, J.; van Eijck, C.H. Long-term survival after radical resection for pancreatic head and ampullary cancer: A potential role for the EGF-R. Dig. Surg. 2007, 24, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Tang, J.; Tudur-Smith, C.; Neoptolemos, J.P.; Ghaneh, P. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br. J. Cancer 2011, 104, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Bassi, C.; Dervenis, C.; Fernandez-Cruz, L.; Lacaine, F.; et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; O’Neill, E.; Gordon-Weeks, A.; Mukherjee, S.; McKenna, W.G.; Muschel, R.J. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochim. Biophys. Acta 2015, 1855, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, M.A.; Kim, T.M.; Lee, S.H.; Kim, D.W.; Im, S.A.; Kim, T.Y.; Kim, W.H.; Yang, H.K.; Heo, D.S.; et al. Biomarker analysis in stage III-IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: Epidermal growth factor receptor (EGFR) associated with favourable survival. Br. J. Cancer 2009, 100, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, P.; Volante, M.; Novello, S.; Rapa, I.; Danenberg, K.D.; Danenberg, P.V.; Cambieri, A.; Selvaggi, G.; Saviozzi, S.; Calogero, R.; et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann. Oncol. 2006, 17, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Vallbohmer, D.; Iqbal, S.; Yang, D.Y.; Rhodes, K.E.; Zhang, W.; Gordon, M.; Fazzone, W.; Schultheis, A.M.; Sherrod, A.E.; Danenberg, K.D.; et al. Molecular determinants of irinotecan efficacy. Int. J. Cancer 2006, 119, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Shostak, K.; Chariot, A. EGFR and NF-κB: Partners in cancer. Trends Mol. Med. 2015, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Yarden, Y. The EGFR-HER2 module: A stem cell approach to understanding a prime target and driver of solid tumors. Oncogene 2016, 35, 2949–2960. [Google Scholar] [CrossRef] [PubMed]
- Kadera, B.E.; Toste, P.A.; Wu, N.; Li, L.; Nguyen, A.H.; Dawson, D.W.; Donahue, T.R. Low expression of the E3 ubiquitin ligase CBL confers chemoresistance in human pancreatic cancer and is targeted by epidermal growth factor receptor inhibition. Clin. Cancer Res. 2015, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Fensterer, H.; Schade-Brittinger, C.; Muller, H.H.; Tebbe, S.; Fass, J.; Lindig, U.; Settmacher, U.; Schmidt, W.E.; Marten, A.; Ebert, M.P.; et al. Multicenter phase II trial to investigate safety and efficacy of gemcitabine combined with cetuximab as adjuvant therapy in pancreatic cancer (ATIP). Ann. Oncol. 2013, 24, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, V.J.; Abrams, R.A.; Decker, P.A.; Traverso, W.; O’Reilly, E.M.; Greeno, E.; Martin, R.C.; Wilfong, L.S.; Rothenberg, M.L.; Posner, M.C.; et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann. Oncol. 2011, 22, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Herman, J.M.; Jimeno, A.; Laheru, D.; Messersmith, W.A.; Wolfgang, C.L.; Cameron, J.L.; Pawlik, T.M.; Donehower, R.C.; Rudek, M.A.; et al. A tolerability and pharmacokinetic study of adjuvant erlotinib and capecitabine with concurrent radiation in resected pancreatic cancer. Transl. Oncol. 2010, 3, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, H.; Essapen, S. Epidermal growth factor receptor inhibitors in cancer treatment: Advances, challenges and opportunities. Anti-Cancer Drug 2009, 20, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Renouf, D.J.; Tang, P.A.; Hedley, D.; Chen, E.; Kamel-Reid, S.; Tsao, M.S.; Tran-Thanh, D.; Gill, S.; Dhani, N.; Au, H.J.; et al. A phase II study of erlotinib in gemcitabine refractory advanced pancreatic cancer. Eur. J. Cancer 2014, 50, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.C.; Morris, P.G. Systemic therapy for advanced pancreatic cancer: Individualising cytotoxic therapy. Expert Opin. Pharmacol. 2015, 16, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Q.; Rosenberg, A.; LoBuglio, A.; Schmidt, W.; Wolff, R.A.; Deutsch, J.; Needle, M.; Abbruzzese, J.L. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II Trial. J. Clin. Oncol. 2004, 22, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef] [PubMed]





| EGFR | Total | Positive vs. Negative | ||
|---|---|---|---|---|
| Negative | Positive | p-Value | ||
| Age, years | ||||
| ≤65 | 231 | 128 | 103 | 0.685 |
| >65 | 126 | 67 | 59 | |
| Gender | ||||
| Male | 205 | 106 | 99 | 0.199 |
| Female | 152 | 89 | 63 | |
| Tumor location | ||||
| Head | 211 | 117 | 94 | 0.705 |
| Others | 146 | 78 | 68 | |
| CA 19-9 (U/mL) | ||||
| ≤37 | 84 | 49 | 35 | 0.435 |
| >37 | 273 | 146 | 127 | |
| Size (cm) | 3.5 ± 1.6 | 3.5 ± 1.7 | 3.5 ± 1.5 | 0.937 |
| Differentiation | ||||
| well | 7 | 6 | 1 | 0.015 |
| moderate | 200 | 119 | 81 | |
| poor | 136 | 61 | 75 | |
| unknown | 14 | 9 | 5 | |
| Tumor stages | ||||
| IA | 24 | 10 | 14 | 0.473 |
| IB | 91 | 54 | 37 | |
| IIA | 89 | 49 | 40 | |
| IIB | 153 | 82 | 71 | |
| Nerve invasion | ||||
| yes | 301 | 169 | 132 | 0.180 |
| no | 56 | 26 | 30 | |
| Vessel invasion | ||||
| yes | 72 | 39 | 33 | 0.931 |
| no | 285 | 156 | 129 | |
| Lymph metastasis | ||||
| yes | 153 | 82 | 71 | 0.736 |
| no | 204 | 113 | 91 | |
| Chemotherapy | ||||
| yes | 258 | 148 | 110 | 0.093 |
| no | 99 | 47 | 52 | |
| Radiotherapy | ||||
| yes | 68 | 48 | 20 | 0.003 |
| no | 289 | 147 | 142 | |
| Ki67 (%) | 34.0 ± 21.6 | 32.4 ± 20.9 | 35.8 ± 22.3 | 0.154 |
| Variables (n) | No. | Median PFS (Months) | p-Value | Median OS (Months) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |||||
| Age, years | ||||||||
| ≤65 | 231 | 10.5 | 0.429 | 14.7 | 1 | 0.082 | - | - |
| >65 | 126 | 11.4 | 13.2 | 1.25 | - | |||
| Gender | ||||||||
| Male | 205 | 10.5 | 0.205 | 13.8 | 1 | 0.204 | - | - |
| Female | 152 | 11.8 | 14.2 | 0.85 | - | |||
| Tumor location | ||||||||
| Head | 211 | 10.8 | 0.696 | 14.0 | 1 | 0.402 | - | - |
| Others | 146 | 11.2 | 13.5 | 0.90 | - | |||
| CA19-9 (U/mL) | ||||||||
| ≤37 | 84 | 12.3 | 0.202 | 17.6 | 1 | 0.039 | 1 | 0.026 |
| >37 | 273 | 10.7 | 13.2 | 1.35 | 1.42 | |||
| Size (cm) | ||||||||
| ≤3 | 178 | 11.8 | 0.172 | 17.6 | 1 | 0.001 | 1 | 0.000 |
| >3 | 179 | 10.3 | 11.0 | 1.61 | 1.67 | |||
| Differentiation | ||||||||
| poor | 136 | 10.4 | 0.209 | 14.5 | 1 | 0.015 | 1 | 0.000 |
| moderate | 200 | 11.1 | 17.7 | 0.705 | 0.716 | |||
| well | 7 | 19.0 | 30.6 | 0.346 | 0.385 | |||
| unknown | 14 | 12.8 | 17.7 | 0.776 | 0.781 | |||
| Tumor stages | ||||||||
| IA | 24 | 14.4 | 0.006 | 21.0 | 1 | 0.000 | 1 | 0.000 |
| IB | 91 | 12.1 | 19.0 | 1.56 | 1.39 | |||
| IIA | 89 | 12.1 | 17.8 | 1.73 | 1.46 | |||
| IIB | 153 | 9.27 | 14.0 | 2.66 | 2.45 | |||
| Nerve invasion | ||||||||
| yes | 301 | 10.7 | 0.144 | 13.8 | 1 | 0.338 | - | - |
| no | 56 | 12.9 | 14.2 | 0.85 | - | |||
| Vessel invasion | ||||||||
| yes | 72 | 9.1 | 0.092 | 9.3 | 1 | 0.002 | 1 | 0.076 |
| no | 285 | 11.3 | 15.0 | 0.63 | 0.75 | |||
| Lymph metastasis | ||||||||
| no | 153 | 9.3 | 0.001 | 16.9 | 1 | 0.000 | 1 | 0.000 |
| yes | 204 | 12.4 | 10.9 | 1.71 | 1.78 | |||
| Chemotherapy | ||||||||
| yes | 258 | 11.6 | 0.113 | 16.1 | 1 | 0.000 | 1 | 0.009 |
| no | 99 | 9.7 | 10.3 | 1.77 | 1.46 | |||
| Radiotherapy | ||||||||
| yes | 68 | 13.1 | 0.062 | 23.3 | 1 | 0.001 | 1 | 0.006 |
| no | 289 | 10.6 | 13.1 | 1.71 | 1.70 | |||
| EGFR | ||||||||
| Negative | 195 | 12.2 | 0.040 | 15.0 | 1 | 0.574 | 1 | 0.986 |
| Positive | 162 | 9.6 | 13.1 | 1.07 | 1.00 | |||
| Characteristics | EGFR (+) | EGFR (−) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |
| Age > 65 | 1.30 | 0.176 | - | - | 1.19 | 0.318 | - | - |
| Female | 0.96 | 0.820 | - | - | 0.79 | 0.165 | - | - |
| Head | 1.10 | 0.605 | - | - | 1.11 | 0.542 | - | - |
| CA19-9 > 37 U/mL | 1.22 | 0.381 | - | - | 1.55 | 0.030 | 1.64 | 0.016 |
| Size > 3 cm | 2.36 | 0.000 | 2.38 | 0.000 | 1.20 | 0.272 | - | - |
| Nerve invasion | 0.69 | 0.112 | - | - | 2.03 | 0.012 | 1.71 | 0.063 |
| Vessel invasion | 1.28 | 0.273 | - | - | 1.99 | 0.000 | 1.67 | 0.015 |
| Lymph metastasis | 1.79 | 0.002 | 1.97 | 0.000 | 1.67 | 0.002 | 1.59 | 0.006 |
| Chemotherapy | 0.47 | 0.000 | 0.54 | 0.002 | 0.70 | 0.061 | 0.80 | 0.280 |
| Radiotherapy | 0.54 | 0.055 | 0.65 | 0.201 | 0.60 | 0.013 | 0.63 | 0.029 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Luo, G.; Liu, C.; Cheng, H.; Lu, Y.; Jin, K.; Liu, Z.; Long, J.; Liu, L.; Xu, J.; et al. The Prognostic and Predictive Role of Epidermal Growth Factor Receptor in Surgical Resected Pancreatic Cancer. Int. J. Mol. Sci. 2016, 17, 1090. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071090
Guo M, Luo G, Liu C, Cheng H, Lu Y, Jin K, Liu Z, Long J, Liu L, Xu J, et al. The Prognostic and Predictive Role of Epidermal Growth Factor Receptor in Surgical Resected Pancreatic Cancer. International Journal of Molecular Sciences. 2016; 17(7):1090. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071090
Chicago/Turabian StyleGuo, Meng, Guopei Luo, Chen Liu, He Cheng, Yu Lu, Kaizhou Jin, Zuqiang Liu, Jiang Long, Liang Liu, Jin Xu, and et al. 2016. "The Prognostic and Predictive Role of Epidermal Growth Factor Receptor in Surgical Resected Pancreatic Cancer" International Journal of Molecular Sciences 17, no. 7: 1090. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071090