Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer
Abstract
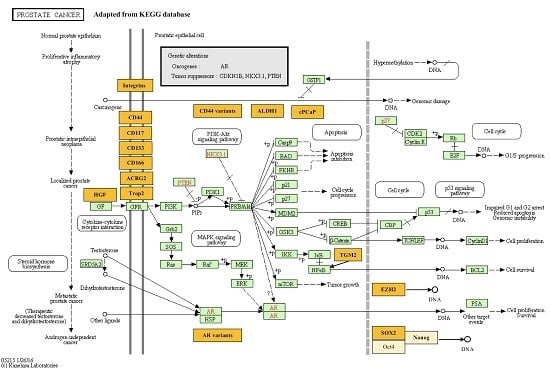
:1. Introduction
2. Integrins
3. CD44
4. CD133
5. ALDH1
6. ATP Binding Membrane Transporters (ABCG2, Also Known as Breast Cancer Resistant Protein or BCRP)
7. SOX2 and EZH2
8. CD166
9. cPAcP
10. Hepatocyte Growth Factor
11. Tumor-Associated Calcium Signal Transducer 2
12. CD117
13. AR Splice Variants
14. TGM2
15. Conclusions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Monsef, N.; Soller, M.; Isaksson, M.; Abrahamsson, P.A.; Panagopoulos, I. The expression of pluripotency marker Oct 3/4 in prostate cancer and benign prostate hyperplasia. Prostate 2009, 69, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate Cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Zong, Y.; Goldstein, A.S. Adaptation or selection—Mechanisms of castration-resistant prostate cancer. Nat. Rev. Urol. 2013, 10, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T.; Coffey, D.S. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981, 41, 5070–5075. [Google Scholar] [PubMed]
- Blum, R.; Gupta, R.; Burger, P.E.; Ontiveros, C.S.; Salm, S.N.; Xiong, X.; Kamb, A.; Wesche, H.; Marshall, L.; Cutler, G.; et al. Molecular signatures of prostate stem cells reveal novel signaling pathways and provide insights into prostate cancer. PLoS ONE 2009, 4, e5722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, S.H.; Frame, F.M.; Collins, A.T. Prostate cancer stem cells. J. Pathol. 2009, 217, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rycaj, K.; Liu, X.; Tang, D.G. New insights into prostate cancer stem cells. Cell Cycle 2013, 12, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.K.; Pellacani, D.; Maitland, N.J. Advanced prostate cancer—A case for adjuvant differentiation therapy. Nat. Rev. Urol. 2012, 9, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Castillo, V.; Valenzuela, R.; Huidobro, C.; Contreras, H.R.; Castellon, E.A. Functional characteristics of cancer stem cells and their role in drug resistance of prostate cancer. Int. J. Oncol. 2014, 45, 985–994. [Google Scholar] [CrossRef] [PubMed]
- McMillen, P.; Holley, S.A. Integration of cell–cell and cell–ECM adhesion in vertebrate morphogenesis. Curr. Opin. Cell Biol. 2015, 36, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Van Slambrouck, S.; Groux-Degroote, S.; Krzewinski-Recchi, M.A.; Cazet, A.; Delannoy, P.; Steelant, W.F. Carbohydrate-to-carbohydrate interactions between α2,3-linked sialic acids on α2 integrin subunits and asialo-GM1 underlie the bone metastatic behaviour of LNCAP-derivative C4-2B prostate cancer cells. Biosci. Rep. 2014, 34. [Google Scholar] [CrossRef] [PubMed]
- Dedhar, S.; Saulnier, R.; Nagle, R.; Overall, C.M. Specific alterations in the expression of α3β1 and α6β4 integrins in highly invasive and metastatic variants of human prostate carcinoma cells selected by in vitro invasion through reconstituted basement membrane. Clin. Exp. Metastasis 1993, 11, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, A.M.; Verhoef, E.I.; Roobol, M.J.; Schroder, F.H.; Wildhagen, M.F.; van der Kwast, T.H.; Jenster, G.; van Leenders, G.J. Validation of stem cell markers in clinical prostate cancer: α 6-integrin is predictive for non-aggressive disease. Prostate 2014, 74, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Habib, F.K.; Maitland, N.J.; Neal, D.E. Identification and isolation of human prostate epithelial stem cells based on α2β1-integrin expression. J. Cell Sci. 2001, 114, 3865–3872. [Google Scholar] [PubMed]
- Fortunel, N.O.; Otu, H.H.; Ng, H.-H.; Chen, J.; Mu, X.; Chevassut, T.; Li, X.; Joseph, M.; Bailey, C.; Hatzfeld, J.A.; et al. Comment on “‘Stemness’: Transcriptional Profiling of Embryonic and Adult Stem Cells” and “A Stem Cell Molecular Signature” (I). Science 2003, 302, 393. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.W.; Axanova, L.S.; Chen, W.; Romero, L.; Maund, S.L.; Soker, S.; Lees, C.J.; Cramer, S.D. Characterization of Adult Prostatic Progenitor/Stem Cells Exhibiting Self-Renewal and Multilineage Differentiation. Stem Cells 2008, 26, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Xin, L.; Lukacs, R.U.; Cheng, D.; Witte, O.N. Isolation and functional characterization of murine prostate stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Marthick, J.R.; Dickinson, J.L. Emerging Putative Biomarkers: The Role of α 2 and 6 Integrins in Susceptibility, Treatment, and Prognosis. Prostate Cancer 2012, 2012, 298732. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Terzuoli, E.; Giachetti, A.; Santi, R.; Villari, D.; Hanaka, H.; Radmark, O.; Ziche, M.; Donnini, S. mPGES-1 in prostate cancer controls stemness and amplifies epidermal growth factor receptor-driven oncogenicity. Endocr. Relat. Cancer 2015, 22, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, B.L.; Lokeshwar, V.B.; Block, N.L. Expression of CD44 in prostate cancer cells: Association with cell proliferation and invasive potential. Anticancer Res. 1995, 15, 1191–1198. [Google Scholar] [PubMed]
- Liu, A.Y. Expression of CD44 in prostate cancer cells. Cancer Lett. 1994, 76, 63–69. [Google Scholar] [CrossRef]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Song, S.; Hochster, H.S.; Steinberg, I.B. Cancer stem cells: The promise and the potential. Semin. Oncol. 2015, 42 (Suppl. S1), S3–S17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Zheng, X.; Wang, X.; Li, S.; Zhang, L.; Yang, Z.; Xia, Z. Enrichment of prostate cancer stem-like cells from human prostate cancer cell lines by culture in serum-free medium and chemoradiotherapy. Int. J. Biol. Sci. 2013, 9, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, Y.; Cui, D.; Li, E.; Zhu, Y.; Zhao, Y.; Zhao, F.; Xia, S. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol. Rep. 2014, 31, 910–918. [Google Scholar] [PubMed]
- Ibuki, N.; Ghaffari, M.; Pandey, M.; Iu, I.; Fazli, L.; Kashiwagi, M.; Tojo, H.; Nakanishi, O.; Gleave, M.E.; Cox, M.E. TAK-441, a novel investigational smoothened antagonist, delays castration-resistant progression in prostate cancer by disrupting paracrine hedgehog signaling. Int. J. Cancer. 2013, 133, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S. Identification, characterization, and biological relevance of prostate cancer stem cells from clinical specimens. Urol. Oncol. 2009, 27, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Van Leenders, G.J.; Schalken, J.A. Stem cell differentiation within the human prostate epithelium: Implications for prostate carcinogenesis. BJU Int. 2001, 88 (Suppl. S2), 35–42. [Google Scholar] [CrossRef] [PubMed]
- Korski, K.; Malicka-Durczak, A.; Breborowicz, J. Expression of stem cell marker CD44 in prostate cancer biopsies predicts cancer grade in radical prostatectomy specimens. Pol. J. Pathol. 2014, 65, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Ugolkov, A.V.; Eisengart, L.J.; Luan, C.; Yang, X.J. Expression analysis of putative stem cell markers in human benign and malignant prostate. Prostate 2011, 71, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.M.; Pontes, J.; Reis, S.T.; Viana, N.I.; Morais, D.R.; Dip, N.; Katz, B.; Srougi, M.; Leite, K.R.M. Expression profile of standard and variants forms of CD44 related to prostate cancer behavior. Int. J. Biol. Markers 2015, 30, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Tei, H.; Miyake, H.; Harada, K.-I.; Fujisawa, M. Expression profile of CD44s, CD44v6, and CD44v10 in localized prostate cancer: Effect on prognostic outcomes following radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Cozzi, P.J.; Hao, J.L.; Beretov, J.; Chang, L.; Duan, W.; Shigdar, S.; Delprado, W.J.; Graham, P.H.; Bucci, J.; et al. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 2014, 74, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Tang, Y.; Habermehl, G.K.; Iczkowski, K.A. Stable alterations of CD44 isoform expression in prostate cancer cells decrease invasion and growth and alter ligand binding and chemosensitivity. BMC Cancer 2010, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Choy, W.; Nagasawa, D.T.; Trang, A.; Thill, K.; Spasic, M.; Yang, I. CD133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme. Neurosurg. Clin. N. Am. 2012, 23, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Gopalan, V.; Wahab, R.; Smith, R.A.; Lam, A.K. Cancer stem cells in oesophageal squamous cell carcinoma: Identification, prognostic and treatment perspectives. Crit. Rev. Oncol./Hematol. 2015, 96, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, A.; Song, H.; Tao, J.; Yang, H.; Zuo, M. Prognostic value of cancer stem cell marker CD133 in ovarian cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 3080–3088. [Google Scholar] [PubMed]
- Han, G.W.; Yi, S.H. Prostate stem cells: An update. Zhonghua Nan Ke Xue 2014, 20, 460–463. [Google Scholar] [PubMed]
- Zenzmaier, C.; Untergasser, G.; Berger, P. Aging of the prostate epithelial stem/progenitor cell. Exp. Gerontol. 2008, 43, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Missol-Kolka, E.; Karbanova, J.; Janich, P.; Haase, M.; Fargeas, C.A.; Huttner, W.B.; Corbeil, D. Prominin-1 (CD133) is not restricted to stem cells located in the basal compartment of murine and human prostate. Prostate 2011, 71, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Jui, H.Y.; Huang, Y.H.; Su, M.Y.; Wu, Y.W.; Tseng, W.Y.; Hsu, M.C.; Chiang, B.L.; Wu, K.K.; Lee, C.M. CXCR4 Antagonist TG-0054 Mobilizes Mesenchymal Stem Cells, Attenuates Inflammation, and Preserves Cardiac Systolic Function in a Porcine Model of Myocardial Infarction. Cell Transplant. 2015, 24, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Rathore, S.; Goel, H.L.; Li, J.; Alberti, S.; Piantelli, M.; Adams, D.; Jiang, Z.; Languino, L.R. CD133, Trop-2 and α2β1 integrin surface receptors as markers of putative human prostate cancer stem cells. Am. J. Transl. Res. 2010, 2, 135–144. [Google Scholar] [PubMed]
- Taylor, R.A.; Toivanen, R.; Frydenberg, M.; Pedersen, J.; Harewood, L.; Australian Prostate Cancer Bioresource; Collins, A.T.; Maitland, N.J.; Risbridger, G.P. Human epithelial basal cells are cells of origin of prostate cancer, independent of CD133 status. Stem Cells 2012, 30, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Xia, P.; Hu, W.Q.; Wang, D.; Xu, X.Y. Aldehyde dehydrogenase 1 expression correlates with clinicopathologic features of patients with breast cancer: A meta-analysis. Int. J. Clinl. Exp. Med. 2015, 8, 8425–8432. [Google Scholar]
- Schnier, J.B.; Kaur, G.; Kaiser, A.; Stinson, S.F.; Sausville, E.A.; Gardner, J.; Nishi, K.; Bradbury, E.M.; Senderowicz, A.M. Identification of cytosolic aldehyde dehydrogenase 1 from non-small cell lung carcinomas as a flavopiridol-binding protein. FEBS Lett. 1999, 454, 100–104. [Google Scholar] [CrossRef]
- Li, T.; Su, Y.; Mei, Y.; Leng, Q.; Leng, B.; Liu, Z.; Stass, S.A.; Jiang, F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab. Investig. J. Tech. Methods Pathol. 2010, 90, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.E.; Gupta, R.; Xiong, X.; Ontiveros, C.S.; Salm, S.N.; Moscatelli, D.; Wilson, E.L. High aldehyde dehydrogenase activity: A novel functional marker of murine prostate stem/progenitor cells. Stem Cells 2009, 27, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Sarkar, F.H.; Wei, W. Targeting prostate cancer stem cells for cancer therapy. Discov. Med. 2012, 13, 135–142. [Google Scholar] [PubMed]
- Gangavarapu, K.J.; Azabdaftari, G.; Morrison, C.D.; Miller, A.; Foster, B.A.; Huss, W.J. Aldehyde dehydrogenase and ATP binding cassette transporter G2 (ABCG2) functional assays isolate different populations of prostate stem cells where ABCG2 function selects for cells with increased stem cell activity. Stem Cell Res. Ther. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Ferronika, P.; Triningsih, F.X.; Ghozali, A.; Moeljono, A.; Rahmayanti, S.; Shadrina, A.N.; Naim, A.E.; Wudexi, I.; Arnurisa, A.M.; Nanwani, S.T.; et al. p63 cytoplasmic aberrance is associated with high prostate cancer stem cell expression. Asian Pac. J. Cancer Prev. (APJCP) 2012, 13, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Ongkeko, W.M. ABCG2: The key to chemoresistance in cancer stem cells? Expert Opin. Drug Metab. Toxicol. 2009, 5, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Zhou, J.; Claypool, K.; Tang, D.G. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005, 65, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Kim, M.C.; Kim, N.Y.; Kim, Y. Inhibition of hedgehog signaling reduces the side population in human malignant mesothelioma cell lines. Cancer Gene Ther. 2015, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Sun, Z.; Wenyong, L.; Dongxia, Y.; Zhao, R.; Zhang, X. A preliminary study of side population cells in human gastric cancer cell line HGC-27. Ann. Transplant. Q. Pol. Transplant. Soc. 2015, 20, 147–153. [Google Scholar]
- Guzel, E.; Karatas, O.F.; Duz, M.B.; Solak, M.; Ittmann, M.; Ozen, M. Differential expression of stem cell markers and ABCG2 in recurrent prostate cancer. Prostate 2014, 74, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.Y.; Hsieh, I.C.; Cheng, J.T.; Tsai, M.H.; Hou, Y.Y.; Lee, J.H.; Liou, H.H.; Huang, S.F.; Chen, H.C.; Yen, L.M.; et al. Association of OCT4, SOX2, and NANOG expression with oral squamous cell carcinoma progression. J. Oral Pathol. Med. 2015, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Li, A.M.; Dunham, C.; Tabori, U.; Carret, A.S.; McNeely, P.D.; Johnston, D.; Lafay-Cousin, L.; Wilson, B.; Eisenstat, D.D.; Jabado, N.; et al. EZH2 expression is a prognostic factor in childhood intracranial ependymoma: A Canadian Pediatric Brain Tumor Consortium study. Cancer 2015, 121, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Hindoyan, A.; Wang, S.; Tran, L.M.; Goldstein, A.S.; Lawson, D.; Chen, D.; Li, Y.; Guo, C.; Zhang, B.; et al. Identification of CD166 as a surface marker for enriching prostate stem/progenitor and cancer initiating cells. PLoS ONE 2012, 7, e42564. [Google Scholar] [CrossRef] [PubMed]
- Rowehl, R.A.; Crawford, H.; Dufour, A.; Ju, J.; Botchkina, G.I. Genomic analysis of prostate cancer stem cells isolated from a highly metastatic cell line. Cancer Genom. Proteom. 2008, 5, 301–310. [Google Scholar]
- Chuang, T.-D.; Chen, S.-J.; Lin, F.-F.; Veeramani, S.; Kumar, S.; Batra, S.K.; Tu, Y.; Lin, M.-F. Human Prostatic Acid Phosphatase, an Authentic Tyrosine Phosphatase, Dephosphorylates ErbB-2 and Regulates Prostate Cancer Cell Growth. J. Biol. Chem. 2010, 285, 23598–23606. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.W.; Lin, F.F.; Muniyan, S.; Lin, F.C.; Chen, C.S.; Wang, J.; Huang, C.C.; Lin, M.F. Cellular prostatic acid phosphatase (cPAcP) serves as a useful biomarker of histone deacetylase (HDAC) inhibitors in prostate cancer cell growth suppression. Cell Biosci. 2015, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.W.; Chaturvedi, N.K.; Ouyang, S.; Lin, F.F.; Kaushik, D.; Wang, J.; Kim, I.; Lin, M.F. Histone deacetylase inhibitor valproic acid suppresses the growth and increases the androgen responsiveness of prostate cancer cells. Cancer Lett. 2011, 311, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Hirohashi, Y.; Torigoe, T.; Nojima, M.; Inoue, R.; Kitamura, H.; Tanaka, T.; Asanuma, H.; Sato, N.; Masumori, N. Expression of hepatocyte growth factor in prostate cancer may indicate a biochemical recurrence after radical prostatectomy. Anticancer Res. 2015, 35, 413–418. [Google Scholar] [CrossRef]
- Fornaro, M.; Dell’Arciprete, R.; Stella, M.; Bucci, C.; Nutini, M.; Capri, M.G.; Alberti, S. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int. J. Cancer 1995, 62, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Ripani, E.; Sacchetti, A.; Corda, D.; Alberti, S. Human Trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer 1998, 76, 671–676. [Google Scholar] [CrossRef]
- Mühlmann, G.; Spizzo, G.; Gostner, J.; Zitt, M.; Maier, H.; Moser, P.; Gastl, G.; Müller, H.M.; Margreiter, R.; Öfner, D.; et al. TROP2 expression as prognostic marker for gastric carcinoma. J. Clin. Pathol. 2009, 62, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Lu, Z.H.; Wang, G.Q.; Pan, Z.Z.; Zhou, Z.W.; Yun, J.P.; Zhang, M.F.; Wan, D.S. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int. J. Colorectal. Dis. 2009, 24, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Shimada, H.; Ochiai, T.; Kuboshima, M.; Kuroiwa, N.; Okazumi, S.; Matsubara, H.; Nomura, F.; Takiguchi, M.; Hiwasa, T. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int. J. Cancer 2004, 112, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ohmachi, T.; Tanaka, F.; Mimori, K.; Inoue, H.; Yanaga, K.; Mori, M. Clinical Significance of TROP2 Expression in Colorectal Cancer. Clin. Cancer Res. 2006, 12, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Moser, P.; Krammel, C.; Gostner, J.M.; Margreiter, R.; Mitterer, M.; Gastl, G.; Spizzo, G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br. J. Cancer 2008, 99, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, e232. [Google Scholar] [CrossRef] [PubMed]
- Höfner, T.; Eisen, C.; Klein, C.; Rigo-Watermeier, T.; Goeppinger, S.M.; Jauch, A.; Schoell, B.; Vogel, V.; Noll, E.; Weichert, W.; et al. Defined Conditions for the Isolation and Expansion of Basal Prostate Progenitor Cells of Mouse and Human Origin. Stem Cell Rep. 2015, 4, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.S.; Lawson, D.A.; Cheng, D.; Sun, W.; Garraway, I.P.; Witte, O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 20882–20887. [Google Scholar] [CrossRef] [PubMed]
- Fedr, R.; Pernicová, Z.; Slabáková, E.; Straková, N.; Bouchal, J.; Grepl, M.; Kozubík, A.; Souček, K. Automatic cell cloning assay for determining the clonogenic capacity of cancer and cancer stem-like cells. Cytom. A 2013, 83, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, T.; Goldstein, A.S.; Cai, H.; Drake, J.M.; Huang, J.; Witte, O.N. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via β-catenin signaling. Genes Dev. 2012, 26, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Jernigan, D.L.; Liu, Q.; Siddiqui, J.; Fatatis, A.; Languino, L.R. Trop-2 promotes prostate cancer metastasis by modulating β1 integrin functions. Cancer Res. 2013, 73, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Li, J.; Alberti, S.; Languino, L.R. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the β1 integrin-RACK1 axis. J. Cell. Physiol. 2012, 227, 3670–3677. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, I.; de Cáceres, I.I.; Hoffman, A.M.; Potapova, A.; Dulaimi, E.; Al-Saleem, T.; Hudes, G.R.; Ochs, M.F.; Cairns, P. Global Reactivation of Epigenetically Silenced Genes in Prostate Cancer. Cancer Prev. Res. 2010, 3, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, C.; Esteller, M. DNA Methylation Markers for Prostate Cancer with a Stem Cell Twist. Cancer Prev. Res. 2010, 3, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Kuo, Y.-C.; Weng, Y.-I.; Lai, I.L.; Huang, T.H.M.; Lin, S.-P.; Niu, D.-M.; Chen, C.-S. Activation of Silenced Tumor Suppressor Genes in Prostate Cancer Cells by a Novel Energy Restriction-Mimetic Agent. Prostate 2012, 72. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, C.M.; Frielink, C.; Goldenberg, D.M.; Sharkey, R.M.; Lutje, S.; McBride, W.J.; Oyen, W.J.; Boerman, O.C. Pretargeted Radioimmunotherapy of Prostate Cancer with an Anti-TROP-2× Anti-HSG Bispecific Antibody and a 177Lu-Labeled Peptide. Cancer Biother. Radiopharm. 2014, 29, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, C.M.; Lutje, S.; Frielink, C.; Sharkey, R.M.; Goldenberg, D.M.; Franssen, G.M.; McBride, W.J.; Rossi, E.A.; Oyen, W.J.; Boerman, O.C. Pretargeted immuno-PET and radioimmunotherapy of prostate cancer with an anti-TROP-2× anti-HSG bispecific antibody. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Kuang, W.J.; Yang-Feng, T.; Coussens, L.; Munemitsu, S.; Dull, T.J.; Chen, E.; Schlessinger, J.; Francke, U.; Ullrich, A. Human proto-oncogene c-kit: A new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987, 6, 3341–3351. [Google Scholar] [PubMed]
- Matthews, W.; Jordan, C.T.; Wiegand, G.W.; Pardoll, D.; Lemischka, I.R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 1991, 65, 1143–1152. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Maze, R.; Miyazawa, K.; Carow, C.; Hendrie, P.C.; Cooper, S.; Hangoc, G.; Vadhan-Raj, S.; Lu, L. The kit receptor and its ligand, steel factor, as regulators of hemopoiesis. Cancer Cells 1991, 3, 480–487. [Google Scholar] [PubMed]
- Chi, P.; Chen, Y.; Zhang, L.; Guo, X.; Wongvipat, J.; Shamu, T.; Fletcher, J.A.; Dewell, S.; Maki, R.G.; Zheng, D.; et al. ETV1 is a lineage-specific survival factor in GIST and cooperates with KIT in oncogenesis. Nature 2010, 467, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Autorino, R.; D’Armiento, F.P.; Mignogna, C.; de Laurentiis, M.; de Sio, M.; D’Armiento, M.; Damiano, R.; Vecchio, G.; de Placido, S. Expression of proto-oncogene c-kit in high risk prostate cancer. Eur. J. Surg. Oncol. 2004, 30, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Wang, B.E.; Johnson, L.; Gao, W.Q. Generation of a prostate from a single adult stem cell. Nature 2008, 456, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, C.; Nabha, S.M.; Dos Santos, E.B.; Yamamoto, H.; Meng, H.; Melchior, S.W.; Bittinger, F.; Thüroff, J.W.; Vessella, R.L.; Cher, M.L.; et al. C-Kit and Its Ligand Stem Cell Factor: Potential Contribution to Prostate Cancer Bone Metastasis. Neoplasia 2008, 10, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.A.; Miocinovic, R.; Smith, A.K.; West, X.Z.; Watts, K.E.; Alzayed, A.W.; Klink, J.C.; Mir, M.C.; Sturey, T.; Hansel, D.E.; et al. CD117+ cells in the circulation are predictive of advanced prostate cancer. Oncotarget 2015, 6, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Tsuji, K.; Ebihara, Y.; Tanaka, I.; Sawai, N.; Koike, K.; Komiyama, A.; Nakahata, T. Chemotactic and chemokinetic activities of stem cell factor on murine hematopoietic progenitor cells. Blood 1996, 87, 4100–4108. [Google Scholar] [PubMed]
- Blume-Jensen, P.; Claesson-Welsh, L.; Siegbahn, A.; Zsebo, K.M.; Westermark, B.; Heldin, C.H. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991, 10, 4121–4128. [Google Scholar] [PubMed]
- Kong, D.; Sethi, S.; Li, Y.; Chen, W.; Sakr, W.A.; Heath, E.; Sarkar, F.H. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate 2015, 75, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G.; et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion–resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Marcias, G.; Erdmann, E.; Lapouge, G.; Siebert, C.; Barthélémy, P.; Duclos, B.; Bergerat, J.-P.; Céraline, J.; Kurtz, J.-E. Identification of novel truncated androgen receptor (AR) mutants including unreported pre-mRNA splicing variants in the 22Rv1 hormone-refractory prostate cancer (PCa) cell line. Hum. Mutat. 2010, 31, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sprenger, C.C.T.; Vessella, R.L.; Haugk, K.; Soriano, K.; Mostaghel, E.A.; Page, S.T.; Coleman, I.M.; Nguyen, H.M.; Sun, H.; et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Investig. 2010, 120, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Isaacs, W.B.; Luo, J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate 2011, 71, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Chen, Y.F.; Balbas, M.D.; Wongvipat, J.; Socci, N.D.; Viale, A.; Kim, K.; Sawyers, C.L. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 16759–16765. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Schmidt, L.J.; Heemers, H.V.; Vessella, R.L.; Tindall, D.J. Splicing of a Novel Androgen Receptor Exon Generates a Constitutively Active Androgen Receptor that Mediates Prostate Cancer Therapy Resistance. Cancer Res. 2008, 68, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dunn, T.A.; Wei, S.; Isharwal, S.; Veltri, R.W.; Humphreys, E.; Han, M.; Partin, A.W.; Vessella, R.L.; Isaacs, W.B.; et al. Ligand-Independent Androgen Receptor Variants Derived from Splicing of Cryptic Exons Signify Hormone-Refractory Prostate Cancer. Cancer Res. 2009, 69, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Tindall, D.J. Alternatively spliced androgen receptor variants. Endocr. Relat. Cancer 2011, 18, R183–R196. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Antonarakis, E.; Luo, J. Androgen Receptor Splice Variants in the Era of Enzalutamide and Abiraterone. Horm. Cancer 2014, 5, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Onstenk, W.; Sieuwerts, A.M.; Kraan, J.; van, M.; Nieuweboer, A.J.M.; Mathijssen, R.H.J.; Hamberg, P.; Meulenbeld, H.J.; de Laere, B.; Dirix, L.Y.; et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur. Urol. 2015, 68, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Lu, C.; Chen, Y.; Paller, C.J.; Carducci, M.A.; Eisenberger, M.A.; Luo, J.; Antonarakis, E.S. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Han, A.L.; Kumar, S.; Fok, J.Y.; Tyagi, A.K.; Mehta, K. Tissue transglutaminase expression promotes castration-resistant phenotype and transcriptional repression of androgen receptor. Eur. J. Cancer 2014, 50, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Kerr, C. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol. Carcinog. 2015, 54, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Klotz, L.H.; Venkateswaran, V. Metformin and prostate cancer stem cells: A novel therapeutic target. Prostate Cancer Prostatic Dis. 2015, 18, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.Y.; Nguyen, D.; Pajonk, F.; Kupelian, P.; Kaprealian, T.; Selch, M.; Low, D.A.; Sheng, K. Incorporating cancer stem cells in radiation therapy treatment response modeling and the implication in glioblastoma multiforme treatment resistance. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 866–875. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | Transmembrane Protein | Glycoprotein | Enzyme | Transcription Factor | Extracellular Protein | mRNA |
|---|---|---|---|---|---|---|
| Integrins | Yes | - | - | - | - | - |
| CD44 | Yes | - | - | - | - | - |
| CD133 | Yes | Yes | - | - | - | - |
| CD166 | Yes | - | - | - | - | - |
| Trop2 | Yes | Yes | - | - | - | - |
| CD117 | Yes | - | Yes | - | - | - |
| ALDH1 | - | - | - | Yes | - | - |
| ABCG2 | Yes | - | - | - | - | - |
| SOX2 | - | - | - | Yes | - | - |
| EZH2 | - | - | Yes | - | - | - |
| cPAcP | - | - | Yes | - | - | - |
| AR splice variants | - | - | - | - | - | Yes |
| HGF | - | - | - | - | Yes | - |
| TGM2 | - | - | Yes | - | - | - |
| Markers | PCa Cell Lines | Primary PCa Tissues | Mouse Models | Possible Involved Pathway in PCa |
|---|---|---|---|---|
| Integrins | Yes | Yes | - | - |
| CD44 | Yes | Yes | - | - |
| CD133 | Yes | Yes | - | - |
| CD166 | - | - | Yes | - |
| Trop2 | - | - | Yes | - |
| CD117 | - | - | Yes | - |
| ALDH1 | Yes | - | - | - |
| ABCG2 | Yes | Yes | - | - |
| SOX2 | - | Yes | - | - |
| EZH2 | - | Yes | - | - |
| cPAcP | Yes | - | - | - |
| AR splice variants | Yes | - | - | AR |
| HGF | Yes | - | - | AR |
| TGM2 | Yes | - | - | NF-κB |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhou, S.; Wang, L.; Wang, J.; Zou, Q.; Zhao, W.; Fu, Q.; Fang, X. Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 1163. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071163
Zhang K, Zhou S, Wang L, Wang J, Zou Q, Zhao W, Fu Q, Fang X. Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer. International Journal of Molecular Sciences. 2016; 17(7):1163. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071163
Chicago/Turabian StyleZhang, Kaile, Shukui Zhou, Leilei Wang, Jianlong Wang, Qingsong Zou, Weixin Zhao, Qiang Fu, and Xiaolan Fang. 2016. "Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer" International Journal of Molecular Sciences 17, no. 7: 1163. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071163