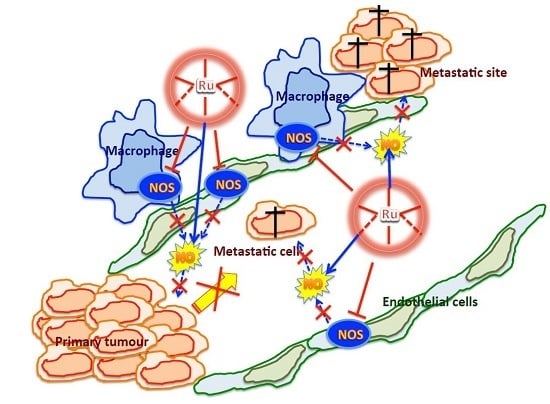
Pharmacological Activities of Ruthenium Complexes Related to Their NO Scavenging Properties
Abstract
:1. Introduction
2. Results
2.1. Murine Peritoneal Macrophage Model
Effects on Nitric Oxide (NO) Production
2.2. EA.Hy926 Endothelial Cell Line Model
2.2.1. Cytotoxicity Test
2.2.2. Cell Invasion Test
2.2.3. NO Production and Release
2.3. Anti-Angiogenic Activity of NAMI-A in the Model of Matrigel™ Pellets
3. Discussion
4. Materials and Methods
4.1. Compounds and Chemicals
4.2. Murine Peritoneal Macrophages
4.3. EA.hy926 Cell Line
4.4. Cytotoxicity Test
4.5. Invasion Assay
4.6. Nitric Oxide Measurements
4.7. Griess Test
4.8. DAF-2 DA Fluorescent Probe
4.9. Matrigel™ Pellets Angiogenic Test
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, A.; Chiodelli, P.; Matarazzo, S.; Rusnati, M.; Presta, M. Blocking the FGF/FGFR system as a “two-compartment” antiangiogenic/antitumor approach in cancer therapy. Pharmacol. Res. 2016, 107, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Bevacizumab: A review of its use in advanced cancer. Drugs 2014, 74, 1891–1925. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.P.; Goodman, V.; Jiang, J.X.; Mahjoob, K.; Verbois, S.L.; Morse, D.; Dagher, R.; Justice, R.; Pazdur, R. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist 2007, 12, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sherbet, G.V. Therapeutic potential of thalidomide and its analogues in the treatment of cancer. Anticancer Res. 2015, 35, 5767–5772. [Google Scholar] [PubMed]
- Vacca, A.; Bruno, M.; Boccarelli, A.; Coluccia, M.; Ribatti, D.; Bergamo, A.; Garbisa, S.; Sartor, L.; Sava, G. Inhibition of endothelial cell functions and of angiogenesis by the metastasis inhibitor NAMI-A. Br. J. Cancer 2002, 86, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L.; Donnini, S.; Filippi, S.; Messori, L.; Piccioli, F.; Orioli, P.; Sava, G.; Ziche, M. Anti angiogenic properties of selected ruthenium(III) complexes that are nitric oxide scavengers. Br. J. Cancer 2003, 88, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Sanna, B.; Dedidda, M.; Pintus, G.; Tadolini, B.; Posadino, A.M.; Bennardini, F.; Sava, G.; Ventura, C. The anti-metastatic agent imidazolium trans-imidazoledimethylsulfoxide-tetrachlororuthenate induces endothelial cell apoptosis by inhibiting the mitogen-activated protein kinase/extracellular signal-regulated kinase signalling pathway. Arch. Biochem. Biophys. 2002, 403, 209–218. [Google Scholar] [CrossRef]
- Pintus, G.; Tadolini, B.; Posadino, A.M.; Sanna, B.; Debidda, M.; Bennardini, F.; Sava, G.; Ventura, C. Inhibition of the MEK/ERK signalling pathway by the novel antimetastatic agent NAMI-A down regulates c-myc gene expression and endothelial cell proliferation. Eur. J. Biochem. 2002, 269, 5861–5870. [Google Scholar] [CrossRef] [PubMed]
- Cocchietto, M.; Sava, G. Blood concentration and toxicity of the antimetastasis agent NAMI-A following repeated intravenous treatment in mice. Pharmacol. Toxicol. 2000, 87, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Masini, B.; Amerini, S.; Granger, H.J.; Maggi, C.A.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994, 94, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Choudhuri, R.; Zhang, H.T.; Donnini, S.; Granger, H.J.; Bicknell, R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Investig. 1997, 99, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; Morbidelli, L.; Cui, X.L.; Douglas, J.G.; Hood, J.; Granger, H.J.; Ledda, F.; Ziche, M. Nitric oxide is an upstream signal for vascular endothelial growth factor-induced extracellular signal-regulated kinases1/2 activation in postcapillary endothelium. J. Biol. Chem. 1998, 273, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Krol, W.; Czuba, Z.P.; Threadgill, M.D.; Cunningham, B.D.M.; Pietsz, G. Inhibition of nitric oxide (NO·) production in murine macrophages by flavones. Biochem. Parmacol. 1995, 50, 1031–1035. [Google Scholar] [CrossRef]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridisation. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- Keppler, B.K.; Henn, M.; Juhl, U.M.; Berger, M.R.; Niebl, R.; Wagner, F.E. New ruthenium complexes for the treatment of cancer. In Progress in Clinical Biochemistry and Medicine; Springer: Berlin, Germany, 1989; Volume 10, pp. 41–69. [Google Scholar]
- Sava, G.; Pacor, S.; Zorzet, S.; Alessio, E.; Mestroni, G. Antitumour properties of dimethylsulphoxide ruthenium(II) complexes in the Lewis Lung Carcinoma system. Pharmacol. Res. 1989, 21, 617–628. [Google Scholar] [CrossRef]
- Sava, G.; Pacor, S.; Mestroni, G.; Alessio, E. Effects of the Ru(III) complexes [mer-RuCl3(DMSO)2Im][degrees] and Na[trans-RuCl4(DMSO)Im] on solid mouse tumors. Anti-Cancer Drugs 1992, 3, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Pacor, S.; Bergamo, A.; Cocchietto, M.; Mestroni, G.; Alessio, E. Effects of ruthenium complexes on experimental tumours: Irrelevance of cytotoxicity for metastasis inhibition. Chem. Biol. Interact. 1995, 95, 109–126. [Google Scholar] [CrossRef]
- Sava, G.; Clerici, K.; Capozzi, I.; Cocchietto, M.; Gagliardi, R.; Alessio, E.; Mestroni, G.; Perbellini, A. Reduction of lung metastasis by ImH[trans-RuCl4(DMSO)Im]: Mechanism of the selective action investigated on mouse tumors. Anti-Cancer Drugs 1999, 10, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Capozzi, I.; Clerici, K.; Gagliardi, R.; Alessio, E.; Mestroni, G. Pharmacological control of lung metastases of solid tumours by a novel ruthenium complex. Clin. Exp. Metastasis 1998, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Zorzet, S.; Turrin, C.; Vita, F.; Soranzo, M.R.; Zabucchi, G.; Cocchietto, M.; Bergamo, A.; DiGiovine, S.; Pezzoni, G.; et al. Dual action of NAMI-A in inhibition of solid tumor metastasis: Selective targeting of metastatic cells and binding to collagen. Clin. Cancer Res. 2003, 9, 1898–1905. [Google Scholar] [PubMed]
- Coluccia, M.; Sava, G.; Salerno, G.; Bergamo, A.; Pacor, S.; Mestroni, G.; Alessio, E. Efficacy of 5-FU combined to Na[trans-RuCl4(DMSO)Im], a novel selective antimetastatic agent, on the survival time of mice with p388 leukemia, P388/DDP subline and MCa mammary carcinoma. Metal-Based Drugs 1995, 2, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Zorzet, S.; Bergamo, A.; Cocchietto, M.; Sorc, A.; Gava, B.; Alessio, E.; Iengo, E.; Sava, G. Lack of in vitro cytotoxicity, associated to increased G2-M cell fraction and inhibition of matrigel invasion, may predict in vivo-selective antimetastasis activity of ruthenium complexes. J. Pharmacol. Exp. Ther. 2000, 295, 927–933. [Google Scholar] [PubMed]
- Gava, B.; Zorzet, S.; Spessotto, P.; Cocchietto, M.; Sava, G. Inhibition of B16 melanoma metastases with the ruthenium complex imidazolium trans-imidazoledimethylsulfoxide-tetrachlororuthenate and down-regulation of tumor cell invasion. J. Pharmacol. Exp. Ther. 2006, 317, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S.; Budzikowski, A.S.; Tsukahara, H.; Noiri, E. Co-operation between endothelin and nitric oxide in promoting endothelial cell migration and angiogenesis. Clin. Exp. Pharmacol. Physiol. 1999, 26, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Oszajca, M.; Brindell, M.; Stochel, G.; van Eldik, R. Metal-assisted activation of nitric oxide-mechanistic aspecte of complex nitrosylation processes. Adv. Inorg. Chem. 2015, 67, 171–241. [Google Scholar]
- Oszajca, M.; Kulis, E.; Stochel, G.; Brindell, M. Interaction of the NAMI-A complex with nitric oxide under physiological conditions. New J. Chem. 2014, 38, 3386–3394. [Google Scholar] [CrossRef]
- Bucinsky, L.; Büchel, G.E.; Ponec, R.; Rapta, P.; Breza, M.; Kozisek, J.; Gall, M.; Biskupic, S.; Fronc, M.; Schiessl, K.; et al. On the electronic structure of mer,trans-[RuCl3(1H-indazole)2(NO)], a hypothetical metabolite of the antitumor drug candidate KP1019: An experimental and DFT study. Eur. J. Inorg. Chem. 2013, 2013, 2505–2519. [Google Scholar] [CrossRef]
- Das, D.; Mondal, P. Quantum chemical studies on detail mechanism of nitrosylation of NAMI-A-HSA adduct. J. Phys. Chem. 2015, 119, 10456–10465. [Google Scholar] [CrossRef] [PubMed]
- Aitken, J.B.; Antony, S.; Weekley, C.M.; Lai, B.; Spiccia, L.; Harris, H.H. Distinct cellular fates for KP1019 and NAMI-A determined by X-ray fluorescence imaging of single cells. Metallomics 2012, 4, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Rademaker-Lakhai, J.M.; van den Bongard, D.; Pluim, D.; Beijnen, J.H.; Schellens, J.H. A Phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin. Cancer Res. 2004, 10, 3717–3727. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Gerdol, M.; Lucafò, M.; Pelillo, C.; Battaglia, M.; Pallavicini, A.; Sava, G. RNA-seq analysis of the whole transcriptome of MDA-MB-231 mammary carcinoma cells exposed to the antimetastatic drug NAMI-A. Metallomics 2015, 7, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Mestroni, G.; Alessio, E.; Sava, G. New Salt of Anionic Complexes of Ru(III) as Antimetastatic and Antineoplastic Agents. International Patent WO 98/00431, 1 August 1998. [Google Scholar]
- Diamantis, A.A.; Dubrawsky, J.V. Preparation and structure of ethylenediaminetetraacetate complexes of ruthenium(II) with dinitrogen, carbon monoxide, and other π-acceptor ligands. Inorg. Chem. 1981, 20, 1142–1150. [Google Scholar] [CrossRef]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Nathan, C.F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 1989, 169, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ma, F.H.; Hoshi, H.; Oka, M.; Noda, K.; Ukai, Y.; Kojima, H.; Nagano, T.; Toda, N. Determination and bioimaging method for nitric oxide in biological specimens by diaminofluorescein fluorimetry. Anal. Biochem. 2000, 287, 203–209. [Google Scholar]
- Nakatsubo, N.; Kojima, H.; Kikuchi, K.; Nagoshi, H.; Hirata, Y.; Maeda, D.; Imai, Y.; Irimura, T.; Nagano, T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: Diaminofluoresceins. FEBS Lett. 1998, 427, 263–266. [Google Scholar] [CrossRef]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric studies II. Preparations from washed blood cells; nitric oxide haemoglobin and sulfhemoglobin. J. Biol. Chem. 1935, 112, 51–55. [Google Scholar]














| Compound | IC50 [μM] | |
|---|---|---|
| 48 h | 72 h | |
| NAMI-A | 960 (623–1481) | 360 (150–885) |
| RuEDTA | >1000 | 880 (507–1534) |
| KP1339 | 37 (20–68) | 22 (12–38) |
| PTIO | 87 | 94 |
| SNAP | 500 (85–2893) | 220 (85–599) |
| l-NAME | >1000 | >1000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellarin, A.; Zorzet, S.; Bergamo, A.; Sava, G. Pharmacological Activities of Ruthenium Complexes Related to Their NO Scavenging Properties. Int. J. Mol. Sci. 2016, 17, 1254. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081254
Castellarin A, Zorzet S, Bergamo A, Sava G. Pharmacological Activities of Ruthenium Complexes Related to Their NO Scavenging Properties. International Journal of Molecular Sciences. 2016; 17(8):1254. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081254
Chicago/Turabian StyleCastellarin, Anna, Sonia Zorzet, Alberta Bergamo, and Gianni Sava. 2016. "Pharmacological Activities of Ruthenium Complexes Related to Their NO Scavenging Properties" International Journal of Molecular Sciences 17, no. 8: 1254. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081254