Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice
Abstract
:1. Introduction
2. Results
2.1. Body Weight Gain, sGOT, sGPT, Triglyceride (TG) and Glucose in Serum
2.2. Hematoxylin and Eosin (H&E) Staining of Livers
2.3. Immunohistochemistry (IHC) Staining of TNFα/MMP-9/HSL/p-HSL/ATGL in Steatohepatitis
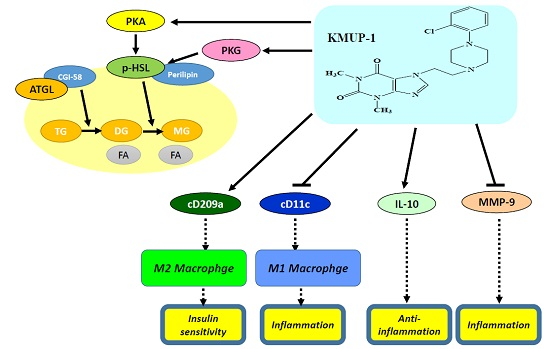
2.4. IHC Staining of Type 1 or Type 2 Macrophages (M1 or M2) in Steatohepatitis
2.5. Expression of IL-10 and MMP-9 in HFD Livers
2.6. Effects of Hyperadiposity on Hepatic Reactive Oxygen Species (ROS)
3. Discussion
4. Materials and Methods
4.1. Animals and Blood Sampling
4.2. Measurement of Hepatic Oil Globules Diameter
4.3. Hematoxylin-Eosin (H&E) Staining of Liver Tissues
4.4. Immunohistochemistry (IHC) Staining of Liver Tissues and Macrophages
4.5. Western Blotting Analysis in Liver Tissues
4.6. Measurement of Hepatic ROS
4.7. Statistical Evaluation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuo, K.K.; Wu, B.N.; Liu, C.P.; Yang, T.Y.; Kao, L.P.; Wu, J.R.; Lai, W.T.; Chen, I.J. Xanthine-based KMUP-1 improves HDL via PPARγ/SR-B1, LDL via LDLRs, and HSL via PKA/PKG for hepatic fat loss. J. Lipid Res. 2015, 56, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Dai, Z.K.; Huang, C.H.; Yeh, J.L.; Wu, B.N.; Wu, J.R.; Chen, I.J. Endothelial nitric oxide synthase-enhancing G-protein coupled receptor antagonist inhibits pulmonary artery hypertension by endothelin-1-dependent and endothelin-1-independent pathways in a monocrotaline model. Kaohsiung J. Med. Sci. 2014, 30, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.K.; Lin, T.C.; Liou, J.C.; Cheng, K.I.; Chen, J.Y.; Chu, L.W.; Chen, I.J.; Wu, B.N. Xanthine derivative KMUP-1 reduces inflammation and hyperalgesia in a bilateral chronic constriction injury model by suppressing MAPK and NFκB activation. Mol. Pharm. 2014, 11, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.F.; Hsu, J.H.; Chen, Y.T.; Chen, I.J.; Yeh, J.L. KMUP-1 attenuates endothelin-1-induced cardiomyocyte hypertrophy through activation of heme oxygenase-1 and suppression of the Akt/GSK-3β, calcineurin/NFATc4 and RhoA/ROCK pathways. Molecules 2015, 20, 10435–10449. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Dai, Z.K.; Wu, B.N.; Yeh, J.L.; Chai, C.Y.; Chu, K.S.; Liu, C.P.; Chen, I.J. KMUP-1 inhibits pulmonary artery proliferation by targeting serotonin receptors/transporter and NO synthase, inactivating RhoA and suppressing AKT/ERK phosphorylation. Vascul. Pharmacol. 2010, 53, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Dai, Z.K.; Wu, B.N.; Yeh, J.L.; Chai, C.Y.; Chu, K.S.; Liu, C.P.; Chen, I.J. The xanthine derivative KMUP-1 inhibits models of pulmonary artery hypertension via increased NO and cGMP-dependent inhibition of RhoA/Rho kinase. Br. J. Pharmacol. 2010, 160, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Sohle, J.; Knott, A.; Holtzmann, U.; Siegner, R.; Gronniger, E.; Schepky, A.; Gallinat, S.; Wenck, H.; Stab, F.; Winnefeld, M. White Tea extract induces lipolytic activity and inhibits adipogenesis in human subcutaneous (pre)-adipocytes. Nutr. Metab. (Lond.) 2009, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Moro, C.; Berlan, M.; Crampes, F.; Sengenes, C.; Galitzky, J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 2008, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, J.; Yan, G.; Wu, J.; Sun, X.; Shen, J.; Jiang, H.; Wang, H. Sildenafil promotes adipogenesis through a PKG pathway. Biochem. Biophys. Res. Commun. 2010, 396, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Marzolla, V.; Rosano, G.M.; Fabbri, A.; Caprio, M. Phosphodiesterase type 5 (PDE5) in the adipocyte: A novel player in fat metabolism? Trends Endocrinol. Metab. 2011, 22, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Osuga, J.; Yahagi, N.; Okazaki, H.; Tamura, Y.; Igarashi, M.; Takase, S.; Harada, K.; Okazaki, S.; Iizuka, Y.; et al. Hormone-sensitive lipase is involved in hepatic cholesteryl ester hydrolysis. J. Lipid Res. 2008, 49, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Osmond, R.I.; Crouch, M.F.; Dupriez, V.J. An emerging role for kinase screening in GPCR drug discovery. Curr. Opin. Mol. Ther. 2010, 12, 305–315. [Google Scholar] [PubMed]
- Wouters, K.; van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Lutjohann, D.; Kerksiek, A.; van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.K.; Wu, B.N.; Chiu, E.Y.; Tseng, C.J.; Yeh, J.L.; Liu, C.P.; Chai, C.Y.; Chen, I.J. NO donor KMUP-1 improves hepatic ischemia-reperfusion and hypoxic cell injury by inhibiting oxidative stress and pro-inflammatory signaling. Int. J. Immunopathol. Pharmacol. 2013, 26, 93–106. [Google Scholar] [PubMed]
- Wang, X.; Liu, J.Z.; Hu, J.X.; Wu, H.; Li, Y.L.; Chen, H.L.; Bai, H.; Hai, C.X. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radic. Biol. Med. 2011, 51, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Somech, R.; Brazovski, E.; Reich, R.; Belson, A.; Konikoff, F.M.; Kessler, A. Matrix metalloproteinases 2 and 9 are markers of inflammation but not of the degree of fibrosis in chronic hepatitis C. Digestion 2005, 71, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Masaki, T.; Seike, M.; Yoshimatsu, H. TNF-α induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp. Biol. Med. (Maywood) 2007, 232, 614–621. [Google Scholar] [PubMed]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hart, E.; Shchurin, A.; Hoover-Plow, J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J. Clin. Investig. 2008, 118, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 Macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Osaki, N.; Shimotoyodome, A. Green tea catechins enhance norepinephrine-induced lipolysis via a protein kinase A-dependent pathway in adipocytes. Biochem. Biophys. Res. Commun. 2015, 461, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery oflipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [PubMed]
- Stienstra, R.; Duval, C.; Muller, M.; Kersten, S. PPARs, obesity, and inflammation. PPAR Res. 2007, 2007, 95974. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.-N.; Kuo, K.-K.; Chen, Y.-H.; Chang, C.-T.; Huang, H.-T.; Chai, C.-Y.; Dai, Z.-K.; Chen, I.-J. Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice. Int. J. Mol. Sci. 2016, 17, 1345. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081345
Wu B-N, Kuo K-K, Chen Y-H, Chang C-T, Huang H-T, Chai C-Y, Dai Z-K, Chen I-J. Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice. International Journal of Molecular Sciences. 2016; 17(8):1345. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081345
Chicago/Turabian StyleWu, Bin-Nan, Kung-Kai Kuo, Yu-Hsun Chen, Chain-Ting Chang, Hung-Tu Huang, Chee-Yin Chai, Zen-Kong Dai, and Ing-Jun Chen. 2016. "Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice" International Journal of Molecular Sciences 17, no. 8: 1345. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081345