Plant Defensins NaD1 and NaD2 Induce Different Stress Response Pathways in Fungi
Abstract
:1. Background
2. Results
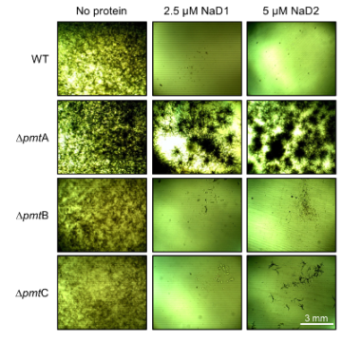
2.1. Impact of Plant Defensins, NaD1 and NaD2, on the Growth of Aspergillus nidulans (A. nidulans) O-Mannosyltransferase Knockout (KO) Mutants
2.2. Defensin Growth Inhibition of the Fusarium oxyspoprum f. sp. lycopersici Mitogen-Activated Protein Kinase (MAPK) Pathway KO Mutants
3. Discussion
4. Methods
4.1. Fungal Strains and Media
4.2. Protein Source
4.3. Fungal Growth Assays
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lay, F.T.; Anderson, M.A. Defensins-components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Schirra, H.J.; Scanlon, M.J.; Anderson, M.A.; Craik, D.J. The three-dimensional solution structure of NaD1, a new floral defensin from Nicotiana alata and its application to a homology model of the crop defense protein alfAFP. J. Mol. Biol. 2003, 325, 175–188. [Google Scholar] [CrossRef]
- Lacerda, A.F.; Vasconcelos, E.A.; Pelegrini, P.B.; Grossi de Sa, M.F. Antifungal defensins and their role in plant defense. Front. Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dracatos, P.M.; van der Weerden, N.L.; Carroll, K.T.; Johnson, E.D.; Plummer, K.M.; Anderson, M.A. Inhibition of cereal rust fungi by both class I and II defensins derived from the flowers of Nicotiana alata. Mol. Plant Pathol. 2014, 15, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Hancock, R.E.W.; Anderson, M.A. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J. Biol. Chem. 2010, 285, 37513–37520. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.M.; Bleackley, M.R.; Wiltshire, J.L.; Anderson, M.A.; Traven, A.; van der Weerden, N.L. Identification and mechanism of action of the plant defensin NaD1 as a new member of the antifungal drug arsenal against Candida albicans. Antimicrob. Agents Chemother. 2013, 57, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.; Payne, J.; Hayes, B.; Durek, T.; Craik, D.; Shafee, T.; Poon, I.; Hulett, M.; Weerden, N.v.d.; Anderson, M. Nicotiana alata defensin chimeras reveal differences in the mechanism of fungal and tumour cell killing and an enhanced antifungal variant. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef]
- Payne, J.A.; Bleackley, M.R.; Lee, T.H.; Shafee, T.M.; Poon, I.K.; Hulett, M.D.; Aguilar, M.I.; van der Weerden, N.L.; Anderson, M.A. The plant defensin NaD1 introduces membrane disorder through a specific interaction with the lipid, phosphatidylinositol 4,5-bisphosphate. Biochim. Biophys. Acta 2016, 1858, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.; Baxter, A.A.; Lay, F.T.; Mills, G.D.; Adda, C.G.; Payne, J.A.; Phan, T.K.; Ryan, G.F.; White, J.A.; Veneer, P.K.; et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. eLife 2014. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.C.; Lee, B.; Young, M.E.; Koo, S.C.; Cooper, J.A.; Baek, D.; Lim, C.O.; Lee, S.Y.; Yun, D.-J.; Cho, M.J. Pn-amp1, a plant defense protein, induces actin depolarization in yeasts. Plant Cell. Physiol 2004, 45, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Mora-Montes, H.M.; Gow, N.A.R.; Coote, P.J. Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface. Microbiology 2009, 155, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hamaguchi, T.; Sameshima, Y.; Goto, M.; Furukawa, K. Molecular characterization of protein O-mannosyltransferase and its involvement in cell-wall synthesis in Aspergillus nidulans. Microbiology 2004, 150, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Harada, Y.; Oka, T.; Matsumoto, S.; Takegawa, K.; Furukawa, K. Protein O-mannosyltransferases B and C support hyphal development and differentiation in Aspergillus nidulans. Eukaryot. Cell 2009, 8, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.D.; Momany, M. Aspergillus nidulans polarity mutant swoA is complemented by protein O-mannosyltransferase pmta. Fungal Genet. Biol. 2002, 37, 263–270. [Google Scholar] [CrossRef]
- Perez-Nadales, E.; di Pietro, A. The membrane mucin Msb2 regulates invasive growth and plant infection in Fusarium oxysporum. Plant Cell 2011, 23, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.C.; Albertyn, J.; Alexander, M.; Davenport, K. Map kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1998, 62, 1264–1300. [Google Scholar] [PubMed]
- Heilmann, C.J.; Sorgo, A.G.; Mohammadi, S.; Sosinska, G.J.; de Koster, C.G.; Brul, S.; de Koning, L.J.; Klis, F.M. Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryot. Cell 2013, 12, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Peña, J.M.; García, R.; Nombela, C.; Arroyo, J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: A yeast dialogue between mapk routes. Yeast 2010, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; de Mello Tavares, P.; Xu, D.; Blankenship, J.; Vandenbosch, D.; Idkowiak-Baldys, J.; Govaert, G.; Bink, A.; Rozental, S.; de Groot, P.W.J.; et al. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 2012, 84, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Zhao, X.; Snyder, A.K.; Xu, J.R.; Shah, D.M. Two mitogen-activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell. Microbiol. 2007, 9, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Lay, F.T.; Anderson, M.A. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J. Biol. Chem. 2008, 283, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, L.N.; Angeli, R.; Sarzedas, C.G.; Barreto-Bergter, E.; Valente, A.P.; Kurtenbach, E.; Almeida, F.C.L. Backbone dynamics of the antifungal PsD1 pea defensin and its correlation with membrane interaction by NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2010, 1798, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sagaram, U.S.; El-Mounadi, K.; Buchko, G.W.; Berg, H.R.; Kaur, J.; Pandurangi, R.S.; Smith, T.J.; Shah, D.M. Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: Identification of an RGFRRR motif governing fungal cell entry. PLoS ONE 2013, 8, e82485. [Google Scholar] [CrossRef] [PubMed]
- De Paula, V.S.; Razzera, G.; Barreto-Bergter, E.; Almeida, F.C.L.; Valente, A.P. Portrayal of complex dynamic properties of sugarcane defensin 5 by NMR: Multiple motions associated with membrane interaction. Structure 2011, 19, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kriangkripipat, T.; Momany, M. Aspergillus nidulans Pmts form heterodimers in all pairwise combinations. FEBS Open Biol. 2014, 4, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Futagami, T.; Nakao, S.; Kido, Y.; Oka, T.; Kajiwara, Y.; Takashita, H.; Omori, T.; Furukawa, K.; Goto, M. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryot. Cell 2011, 10, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Argimon, S.; Fanning, S.; Blankenship, J.R.; Mitchell, A.P. Interaction between the Candida albicans high-osmolarity glycerol (HOG) pathway and the response to Human β-Defensins 2 and 3. Eukaryot. Cell 2011, 10, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Jang, W.S.; Li, W.; Nayyar, N.; Edgerton, M. Histatin 5 initiates osmotic stress response in Candida albicans via activation of the HOG1 mitogen-activated protein kinase pathway. Eukaryot. Cell 2007, 6, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.R.; Wiltshire, J.L.; Perrine-Walker, F.; Vasa, S.; Burns, R.L.; van der Weerden, N.L.; Anderson, M.A. Agp2p, the plasma membrane transregulator of polyamine uptake, regulates the antifungal activities of the plant defensin NaD1 and other cationic peptides. Antimicrob. Agents Chemother. 2014, 58, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Kriangkripipat, T.; Momany, M. Aspergillus nidulans protein O-mannosyltransferases play roles in cell wall integrity and developmental patterning. Eukaryot. Cell 2009, 8, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Garcia-MacEira, F.I.; Meglecz, E.; Roncero, M.I. A map kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.S.; di Pietro, A.; Pérez-Nadales, E.; Turrà, D. Three Fusarium. oxysporum MAPKs have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol. Plant Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Turra, D.; El Ghalid, M.; Rossi, F.; di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Rispail, N.; di Pietro, A. The two-component histidine kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 395–407. [Google Scholar] [CrossRef] [PubMed]


| Fungal Species | Genotype/Phenotype | Protein | Source or Reference |
|---|---|---|---|
| Aspergillus nidulans | Wild Type A850-arg::trpC_B methG | – | Kriangkripipat and Momany [32] |
| ATK08-pyrG89 argB2::trpC B pyroA4 ΔpmtA::AfpyrG | O-mannosyltransferase subfamily pmtA | Kriangkripipat and Momany [32] | |
| ATK16-pyrG89 ΔpmtB::AfpyrG argB2 pyroA4 | O-mannosyltransferase subfamily pmtB | Kriangkripipat and Momany [32] | |
| ATK38-pyrG89 wA3 argB2 pyro A4 ΔpmtC::AfpyrG | O-mannosyltransferase subfamily pmtC | Kriangkripipat and Momany [32] | |
| F. oxysporum f. sp. lycopersici | race 2 wild-type strain 4287 (FGSC 9935) | – | Di Pietro, et al. [33] |
| Δhog1 | High osmolarity glycerol MAPK | Luque et al. [34] | |
| Δmsb2 | Glycosylated receptor of the HOG pathway | Perez-Nadales and Di Pietro [15] | |
| Δmsb2 + Msb2 | Complemented Msb2 | Perez-Nadales and Di Pietro [15] | |
| Δmpk1 | Cell wall integrity MAPK | Turra, et al. [35] | |
| Δfmk1 | Pathogenicity MAPK | Di Pietro, Garcia-MacEira, Meglecz and Roncero [33] | |
| Δfhk1 | Histidine kinase | Rispail and Di Pietro [36] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dracatos, P.M.; Payne, J.; Di Pietro, A.; Anderson, M.A.; Plummer, K.M. Plant Defensins NaD1 and NaD2 Induce Different Stress Response Pathways in Fungi. Int. J. Mol. Sci. 2016, 17, 1473. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091473
Dracatos PM, Payne J, Di Pietro A, Anderson MA, Plummer KM. Plant Defensins NaD1 and NaD2 Induce Different Stress Response Pathways in Fungi. International Journal of Molecular Sciences. 2016; 17(9):1473. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091473
Chicago/Turabian StyleDracatos, Peter M., Jennifer Payne, Antonio Di Pietro, Marilyn A. Anderson, and Kim M. Plummer. 2016. "Plant Defensins NaD1 and NaD2 Induce Different Stress Response Pathways in Fungi" International Journal of Molecular Sciences 17, no. 9: 1473. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17091473