What Is Breast in the Bone?
Abstract
:1. Introduction
2. Breast Cancer Bone Metastases
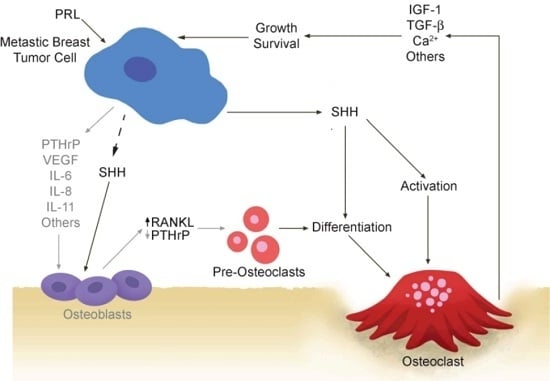
2.1. Vicious Cycle
2.2. Osteoclastogenic Factors Produced by Breast Cancer Cells
2.3. Osteoblastic Factors Produced by Breast Cancer Cells
3. Prolactin in Bone Homeostasis: Pregnancy and Lactation
4. Prolactin and the Regulation of Bone Modulating Factors in the Breast
5. The Role of Prolactin in Breast Cancer-Mediated Osteoclastogenesis
6. Future Directions and Impact: Therapeutic Implications
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CT-1 | cardiotrophin |
| COX-2 | cyclooxygenase-2 |
| ELF5 | E74-like factor 5 (ETS domain transcription factor) |
| EPHB1 | ephrin-B1 |
| ERK | extracellular regulated kinase |
| GSK3β | glycogen synthase kinase-3β |
| JAK2 | Janus kinase-2 |
| HH | hedgehog |
| IGF | insulin-like growth factor |
| IL | interleukin |
| LIGHT | homologous to lymphotoxins exhibiting inducible expression and competing with herpes simplex virus glycoprotein D for herpesvirus entry mediator (HVEM), a receptor expressed by T lymphocytes |
| MCP-1 | monocyte chemoattractant protein-1 |
| M-CSF | macrophage colony stimulating factor |
| MIP3α | macrophage inflammatory protein-3 α |
| NFATc1 | nuclear factor of activated T cells-1 |
| OPG | osteoprotegerin |
| PDGF-BB | platelet-derived growth factor β polypeptide B |
| PKA | protein kinase-A |
| PR | progesterone receptor |
| PRL | prolactin |
| PRLR | prolactin receptor |
| PTCH1 | patched-1 |
| PTHrP | parathyroid hormone-related protein |
| RANKL | receptor activator of nuclear factor-κB (NFκB) ligand |
| SMO | smoothened |
| STAT5a | signal transducer and activator of transcription-5a |
| SUFU | suppressor of fused |
| TGF-β | transforming growth factor-β |
| TNFα | tumor necrosis factor α |
| TRAP | tartrate-resistant acid phosphatase-1 |
| VEGF | vascular endothelial growth factor |
References
- Sutherland, A.; Forsyth, A.; Cong, Y.; Grant, L.; Juan, T.H.; Lee, J.K.; Klimowicz, A.; Petrillo, S.K.; Hu, J.; Chan, A.; et al. The role of prolactin in bone metastasis and breast cancer cell-mediated osteoclast differentiation. J. Natl. Cancer Inst. 2016, 108, djv338. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Kaur, S.; Chavarria, T.E.; Binart, N.; Sutherland, R.L.; Weinberg, R.A.; Kelly, P.A.; Ormandy, C.J. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol. 1999, 210, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Horseman, N.D.; Zhao, W.; Montecino-Rodriguez, E.; Tanaka, M.; Nakashima, K.; Engle, S.J.; Smith, F.; Markoff, E.; Dorshkind, K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997, 16, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Ormandy, C.J.; Camus, A.; Barra, J.; Damotte, D.; Lucas, B.; Buteau, H.; Edery, M.; Brousse, N.; Babinet, C.; Binart, N.; et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997, 11, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.M.; Miyoshi, K.; Robinson, G.W.; Grimm, S.L.; Rosen, J.M.; Neubauer, H.; Pfeffer, K.; Hennighausen, L. JAK2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 2002, 16, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.U.; Krempler, A.; Triplett, A.A.; Qi, Y.; George, N.M.; Zhu, J.; Rui, H. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in JAK2 conditional knockout mice. Mol. Cell. Biol. 2004, 24, 5510–5520. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Riedlinger, G.; Miyoshi, K.; Tang, W.; Li, C.; Deng, C.X.; Robinson, G.W.; Hennighausen, L. Inactivation of STAT5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 2004, 24, 8037–8047. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Shillingford, J.M.; Smith, G.H.; Grimm, S.L.; Wagner, K.U.; Oka, T.; Rosen, J.M.; Robinson, G.W.; Hennighausen, L. Signal transducer and activator of transcription (STAT) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 2001, 155, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, D.; Na, R.; Feuermann, Y.; Pechhold, S.; Chen, W.; Robinson, G.W.; Hennighausen, L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5a. Genes Dev. 2009, 23, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Rose-Hellekant, T.A.; Arendt, L.M.; Schroeder, M.D.; Gilchrist, K.; Sandgren, E.P.; Schuler, L.A. Prolactin induces eralpha-positive and eralpha-negative mammary cancer in transgenic mice. Oncogene 2003, 22, 4664–4674. [Google Scholar] [CrossRef] [PubMed]
- Wennbo, H.; Gebre-Medhin, M.; Gritli-Linde, A.; Ohlsson, C.; Isaksson, O.G.; Tornell, J. Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J. Clin. Investig. 1997, 100, 2744–2751. [Google Scholar] [CrossRef] [PubMed]
- Tikk, K.; Sookthai, D.; Johnson, T.; Rinaldi, S.; Romieu, I.; Tjonneland, A.; Olsen, A.; Overvad, K.; Clavel-Chapelon, F.; Baglietto, L.; et al. Circulating prolactin and breast cancer risk among pre- and postmenopausal women in the epic cohort. Ann. Oncol. 2014, 25, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Tworoger, S.S.; Eliassen, A.H.; Zhang, X.; Qian, J.; Sluss, P.M.; Rosner, B.A.; Hankinson, S.E. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013, 73, 4810–4819. [Google Scholar] [CrossRef] [PubMed]
- Albrektsen, G.; Heuch, I.; Kvale, G. The short-term and long-term effect of a pregnancy on breast cancer risk: A prospective study of 802,457 parous norwegian women. Br. J. Cancer 1995, 72, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Bhatavdekar, J.M.; Patel, D.D.; Shah, N.G.; Vora, H.H.; Suthar, T.P.; Ghosh, N.; Chikhlikar, P.R.; Trivedi, T.I. Prolactin as a local growth promoter in patients with breast cancer: Gcri experience. Eur. J. Surg. Oncol. 2000, 26, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Bhatavdekar, J.M.; Chikhlikar, P.R.; Ghosh, N.; Suthar, T.P.; Shah, N.G.; Mehta, R.H.; Balar, D.B. Node negative breast carcinoma: Hyperprolactinemia and/or overexpression of p53 as an independent predictor of poor prognosis compared to newer and established prognosticators. J. Surg. Oncol. 1996, 62, 86–92. [Google Scholar] [CrossRef]
- Wang, D.Y.; Stepniewska, K.A.; Allen, D.S.; Fentiman, I.S.; Bulbrook, R.D.; Kwa, H.G.; de Stavola, B.L.; Reed, M.J. Serum prolactin levels and their relationship to survival in women with operable breast cancer. J. Clin. Epidemiol. 1995, 48, 959–968. [Google Scholar] [CrossRef]
- Holtkamp, W.; Nagel, G.A.; Wander, H.E.; Rauschecker, H.F.; von Heyden, D. Hyperprolactinemia is an indicator of progressive disease and poor prognosis in advanced breast cancer. Int. J. Cancer 1984, 34, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bhatavdekar, J.M.; Shah, N.G.; Balar, D.B.; Patel, D.D.; Bhaduri, A.; Trivedi, S.N.; Karelia, N.H.; Ghosh, N.; Shukla, M.K.; Giri, D.D. Plasma prolactin as an indicator of disease progression in advanced breast cancer. Cancer 1990, 65, 2028–2032. [Google Scholar] [CrossRef]
- Mujagic, Z.; Mujagic, H. Importance of serum prolactin determination in metastatic breast cancer patients. Croat. Med. J. 2004, 45, 176–180. [Google Scholar] [PubMed]
- Bertucci, F.; Nasser, V.; Granjeaud, S.; Eisinger, F.; Adelaide, J.; Tagett, R.; Loriod, B.; Giaconia, A.; Benziane, A.; Devilard, E.; et al. Gene expression profiles of poor-prognosis primary breast cancer correlate with survival. Hum. Mol. Genet. 2002, 11, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Waseda, N.; Kato, Y.; Imura, H.; Kurata, M. Prognostic value of estrogen and prolactin receptor analysis in human breast cancer. Jpn. J. Cancer Res. 1985, 76, 517–523. [Google Scholar] [PubMed]
- Miller, S.L.; Antico, G.; Raghunath, P.N.; Tomaszewski, J.E.; Clevenger, C.V. Nek3 kinase regulates prolactin-mediated cytoskeletal reorganization and motility of breast cancer cells. Oncogene 2007, 26, 4668–4678. [Google Scholar] [CrossRef] [PubMed]
- Nouhi, Z.; Chughtai, N.; Hartley, S.; Cocolakis, E.; Lebrun, J.J.; Ali, S. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006, 66, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Xie, J.; LeBaron, M.J.; Ealley, E.L.; Nevalainen, M.T.; Rui, H. STAT5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 2005, 24, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tran, T.H.; Peck, A.R.; Liu, C.; Ertel, A.; Lin, J.; Neilson, L.M.; Rui, H. Global profiling of prolactin-modulated transcripts in breast cancer in vivo. Mol. Cancer 2013, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Hachim, I.Y.; Hachim, M.Y.; Lopez, V.M.; Lebrun, J.J.; Ali, S. Prolactin receptor expression is an independent favorable prognostic marker in human breast cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Hachim, I.Y.; Shams, A.; Lebrun, J.J.; Ali, S. A favorable role of prolactin in human breast cancer reveals novel pathway based gene signatures indicative of tumor differentiation and favorable patient outcome: Prolactin-induced mammary differentiation program in breast cancer prognosis. Hum. Pathol. 2016, 53, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Shemanko, C.S. Prolactin receptor in breast cancer: Marker for metastatic risk. J. Mol. Endocrinol. 2016, 57, R153–R165. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.E.; Holt, E.C.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells. PLoS ONE 2015, 10, e0116891. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.E.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J. Biol. Chem. 2013, 288, 12722–12732. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.E.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Prolactin signaling through focal adhesion complexes is amplified by stiff extracellular matrices in breast cancer cells. Oncotarget 2016, 7, 48093–48106. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, E.; Vonderhaar, B.K. Prolactin synthesis and secretion by human breast cancer cells. Cancer Res. 1995, 55, 2591–2595. [Google Scholar] [PubMed]
- Clevenger, C.V. Role of prolactin/prolactin receptor signaling in human breast cancer. Breast Dis. 2003, 18, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ferrag, F.; Chiarenza, A.; Goffin, V.; Kelly, P.A. Convergence of signaling transduced by prolactin (PRL)/cytokine chimeric receptors on PRL-responsive gene transcription. Mol. Endocrinol. 1996, 10, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ferrag, F.; Goffin, V.; Buteau, H.; Kelly, P.A. Immune function of prolactin (PRL) and signal transduction by PRL/GH/cytokine receptors: Specificity, redundancy and lessons from chimaeras. Cytokines Cell. Mol. Ther. 1997, 3, 197–213. [Google Scholar] [PubMed]
- Courtillot, C.; Chakhtoura, Z.; Bogorad, R.; Genestie, C.; Bernichtein, S.; Badachi, Y.; Janaud, G.; Akakpo, J.P.; Bachelot, A.; Kuttenn, F.; et al. Characterization of two constitutively active prolactin receptor variants in a cohort of 95 women with multiple breast fibroadenomas. J. Clin. Endocrinol. Metab. 2010, 95, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, Z.; Laki, F.; Bernadet, M.; Cherifi, I.; Chiche, A.; Pigat, N.; Bernichtein, S.; Courtillot, C.; Boutillon, F.; Bieche, I.; et al. Gain-of-function prolactin receptor variants are not associated with breast cancer and multiple fibroadenoma risk. J. Clin. Endocrinol. Metab. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The science and practice of bone health in oncology: Managing bone loss and metastasis in patients with solid tumors. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. S7), S1–S29. [Google Scholar]
- Akhtari, M.; Mansuri, J.; Newman, K.A.; Guise, T.M.; Seth, P. Biology of breast cancer bone metastasis. Cancer Biol. Ther. 2008, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Bruzzaniti, A.; Baron, R. Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 2006, 7, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Lisignoli, G.; Piacentini, A.; Cristino, S.; Grassi, F.; Cavallo, C.; Cattini, L.; Tonnarelli, B.; Manferdini, C.; Facchini, A. CCL20 chemokine induces both osteoblast proliferation and osteoclast differentiation: Increased levels of CCL20 are expressed in subchondral bone tissue of rheumatoid arthritis patients. J. Cell. Physiol. 2007, 210, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Aoyama, M.; Fukuoka, H.; Fujita, M.; Miyazawa, K.; Asai, K.; Goto, S. IGF2 modulates the microenvironment for osteoclastogenesis. Biochem. Biophys. Res. Commun. 2009, 378, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.C.; McGregor, N.E.; Poulton, I.J.; Pompolo, S.; Allan, E.H.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; Sims, N.A. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J. Bone Miner. Res. 2008, 23, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Inui, M.; Inoue, K.; Kim, S.; Suematsu, A.; Kobayashi, E.; Iwata, T.; Ohnishi, H.; Matozaki, T.; Kodama, T.; et al. Costimulatory signals mediated by the itam motif cooperate with RANKL for bone homeostasis. Nature 2004, 428, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Negishi-Koga, T.; Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009, 231, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med. 2005, 83, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J.; Athanasou, N.A. Canonical and non-canonical pathways of osteoclast formation. Histol. Histopathol. 2009, 24, 337–346. [Google Scholar] [PubMed]
- Powles, T.; Paterson, A.; McCloskey, E.; Schein, P.; Scheffler, B.; Tidy, A.; Ashley, S.; Smith, I.; Ottestad, L.; Kanis, J. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006, 8, R13. [Google Scholar] [CrossRef] [PubMed]
- Gul, G.; Sendur, M.A.; Aksoy, S.; Sever, A.R.; Altundag, K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr. Med. Res. Opin. 2016, 32, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Goessl, C.; Katz, L.; Dougall, W.C.; Kostenuik, P.J.; Zoog, H.B.; Braun, A.; Dansey, R.; Wagman, R.B. The development of denosumab for the treatment of diseases of bone loss and cancer-induced bone destruction. Ann. N. Y. Acad. Sci. 2012, 1263, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Yin, J.J.; Taylor, S.D.; Kumagai, Y.; Dallas, M.; Boyce, B.F.; Yoneda, T.; Mundy, G.R. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Investig. 1996, 98, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, S.E.; Lennard, T.W.; Williams, J.R.; Birch, M.A. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 2005, 335, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Cuervo, C.; Harris, K.W.; Kallman, L.; Vaananen, H.K.; Selander, K.S. Tumor necrosis factor-α induces interleukin-6 production via extracellular-regulated kinase 1 activation in breast cancer cells. Breast Cancer Res. Treat. 2003, 80, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- McCoy, E.M.; Hong, H.; Pruitt, H.C.; Feng, X. IL-11 produced by breast cancer cells augments osteoclastogenesis by sustaining the pool of osteoclast progenitor cells. BMC Cancer 2013, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.J.; Southby, J.; Danks, J.A.; Stillwell, R.G.; Hayman, J.A.; Henderson, M.A.; Bennett, R.C.; Martin, T.J. Localization of parathyroid hormone-related protein in breast cancer metastases: Increased incidence in bone compared with other sites. Cancer Res. 1991, 51, 3059–3061. [Google Scholar] [PubMed]
- Henderson, M.; Danks, J.; Moseley, J.; Slavin, J.; Harris, T.; McKinlay, M.; Hopper, J.; Martin, T. Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J. Natl. Cancer Inst. 2001, 93, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, E.; Jacob, A.P.; Jones, J.; Miller, R.; Roudier-Meyer, M.P.; Erwert, R.; Pinkas, J.; Branstetter, D.; Dougall, W.C. Rank ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 2010, 468, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Schramek, D.; Leibbrandt, A.; Sigl, V.; Kenner, L.; Pospisilik, J.A.; Lee, H.J.; Hanada, R.; Joshi, P.A.; Aliprantis, A.; Glimcher, L.; et al. Osteoclast differentiation factor rankl controls development of progestin-driven mammary cancer. Nature 2010, 468, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, S.E.; Lennard, T.W.; Williams, J.R.; Birch, M.A. Vascular endothelial growth factor acts as an osteolytic factor in breast cancer metastases to bone. Br. J. Cancer 2005, 92, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kaneda, T.; Arakawa, T.; Morita, S.; Sato, T.; Yomada, T.; Hanada, K.; Kumegawa, M.; Hakeda, Y. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000, 473, 161–164. [Google Scholar] [CrossRef]
- Fontanini, G.; Campani, D.; Roncella, M.; Cecchetti, D.; Calvo, S.; Toniolo, A.; Basolo, F. Expression of interleukin 6 (IL-6) correlates with oestrogen receptor in human breast carcinoma. Br. J. Cancer 1999, 80, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, J. Regulation of interleukin-6 secretion from breast cancer cells and its clinical implications. Breast Cancer 2000, 7, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Role of cytokines in bone resorption. Bone 1995, 17, 63S–67S. [Google Scholar] [CrossRef]
- Hernandez, L.L.; Gregerson, K.A.; Horseman, N.D. Mammary gland serotonin regulates parathyroid hormone-related protein and other bone-related signals. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1009–E1015. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Q.; Hu, G.; Van Poznak, C.; Fleisher, M.; Reiss, M.; Massague, J.; Kang, Y. Adamts1 and mmp1 proteolytically engage egf-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009, 23, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kang, Y. Chemokine (C–C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem. 2009, 284, 29087–29096. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Tiedemann, K.; Mourskaia, A.; Fong, J.E.; Tran-Thanh, D.; Amir, E.; Clemons, M.; Perbal, B.; Komarova, S.V.; Siegel, P.M. CCN3 impairs osteoblast and stimulates osteoclast differentiation to favor breast cancer metastasis to bone. Am. J. Pathol. 2011, 178, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordon-Cardo, C.; Guise, T.A.; Massague, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Voorzanger-Rousselot, N.; Goehrig, D.; Journe, F.; Doriath, V.; Body, J.J.; Clezardin, P.; Garnero, P. Increased dickkopf-1 expression in breast cancer bone metastases. Br. J. Cancer 2007, 97, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Mancino, A.T.; Klimberg, V.S.; Yamamoto, M.; Manolagas, S.C.; Abe, E. Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J. Surg. Res. 2001, 100, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Zhang, H.; Zeng, Q.; Dai, J.; Keller, E.T.; Giordano, T.; Gu, K.; Shah, V.; Pei, L.; Zarbo, R.J.; et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat. Med. 2007, 13, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.G.; Sophocleous, A.; Marino, S.; Muir, M.; Brunton, V.G.; Idris, A.I. Selective tyrosine kinase inhibition of insulin-like growth factor-1 receptor inhibits human and mouse breast cancer-induced bone cell activity, bone remodeling, and osteolysis. J. Bone Miner. Res. 2013, 28, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Margulies, A.G.; Walser, B.; Akel, N.S.; Bhattacharrya, S.; Skinner, R.A.; Swain, F.; Ramani, V.; Mohammad, K.S.; Wessner, L.L.; et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-κB ligand pathway. Cancer Res. 2005, 65, 11001–11009. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Lacroix, M.; Lespagnard, L.; Larsimont, D.; Paesmans, M.; Body, J.J. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett. 2001, 169, 87–95. [Google Scholar] [CrossRef]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived jagged1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Eck, S.M.; Hoopes, P.J.; Petrella, B.L.; Coon, C.I.; Brinckerhoff, C.E. Matrix metalloproteinase-1 promotes breast cancer angiogenesis and osteolysis in a novel in vivo model. Breast Cancer Res. Treat. 2009, 116, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Cuervo, C.; Merrell, M.A.; Watson, L.; Harris, K.W.; Rosenthal, E.L.; Vaananen, H.K.; Selander, K.S. Breast cancer cells with inhibition of p38α have decreased MMP-9 activity and exhibit decreased bone metastasis in mice. Clin. Exp. Metastasis 2004, 21, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Bolin, C.; Tawara, K.; Sutherland, C.; Redshaw, J.; Aranda, P.; Moselhy, J.; Anderson, R.; Jorcyk, C.L. Oncostatin m promotes mammary tumor metastasis to bone and osteolytic bone degradation. Genes Cancer 2012, 3, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lev, D.C.; Kim, S.J.; Onn, A.; Stone, V.; Nam, D.H.; Yazici, S.; Fidler, I.J.; Price, J.E. Inhibition of platelet-derived growth factor receptor signaling restricts the growth of human breast cancer in the bone of nude mice. Clin. Cancer Res. 2005, 11, 306–314. [Google Scholar] [PubMed]
- Rafiei, S.; Tiedemann, K.; Tabaries, S.; Siegel, P.M.; Komarova, S.V. Peroxiredoxin 4: A novel secreted mediator of cancer induced osteoclastogenesis. Cancer Lett. 2015, 361, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Buhamrah, A.; Schneider, A.; Lin, Y.L.; Zhou, H.; Bugshan, A.; Basile, J.R. Semaphorin 4d promotes skeletal metastasis in breast cancer. PLoS ONE 2016, 11, e0150151. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Tucker, J.A.; Khullar, S.; Samant, R.S.; Shevde, L.A. Hedgehog signaling in tumor cells facilitates osteoblast-enhanced osteolytic metastases. PLoS ONE 2012, 7, e34374. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Samant, R.S.; Shevde, L.A. Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis. J. Biol. Chem. 2011, 286, 9612–9622. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, M.; Nannuru, K.C.; Varney, M.L.; Sadanandam, A.; Nakao, K.; Asai, K.; Shirai, T.; Sato, S.Y.; Singh, R.K. Transforming growth factor-beta signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci. 2009, 100, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mu, E.; Wei, Y.; Riethdorf, S.; Yang, Q.; Yuan, M.; Yan, J.; Hua, Y.; Tiede, B.J.; Lu, X.; et al. Vcam-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 2011, 20, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Kozlow, W.M.; Heras-Herzig, A.; Padalecki, S.S.; Yin, J.J.; Chirgwin, J.M. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer 2005, 5 (Suppl. S2), S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Williams, P.J.; Niewolna, M.; Wang, Y.; Yoneda, T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 2002, 62, 917–923. [Google Scholar] [PubMed]
- Suntornsaratoon, P.; Wongdee, K.; Goswami, S.; Krishnamra, N.; Charoenphandhu, N. Bone modeling in bromocriptine-treated pregnant and lactating rats: Possible osteoregulatory role of prolactin in lactation. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E426–E436. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Calcium and bone metabolism during pregnancy and lactation. J. Mammary Gland Biol. Neoplasia 2005, 10, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol. Metab. Clin. N. Am. 2011, 40, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Seriwatanachai, D.; Charoenphandhu, N.; Suthiphongchai, T.; Krishnamra, N. Prolactin decreases the expression ratio of receptor activator of nuclear factor κB ligand/osteoprotegerin in human fetal osteoblast cells. Cell Biol. Int. 2008, 32, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Seriwatanachai, D.; Krishnamra, N.; van Leeuwen, J.P. Evidence for direct effects of prolactin on human osteoblasts: Inhibition of cell growth and mineralization. J. Cell. Biochem. 2009, 107, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Seriwatanachai, D.; Thongchote, K.; Charoenphandhu, N.; Pandaranandaka, J.; Tudpor, K.; Teerapornpuntakit, J.; Suthiphongchai, T.; Krishnamra, N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor κB ligand/osteoprotegerin ratio. Bone 2008, 42, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Sun, L.; Liu, P.; Davies, T.F.; New, M.; Zallone, A.; Yuen, T. Pituitary-bone connection in skeletal regulation. Horm. Mol. Biol. Clin. Investig. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Tulalamba, W.; Thongbunchoo, J.; Krishnamra, N.; Charoenphandhu, N. Prolactin alters the mRNA expression of osteoblast-derived osteoclastogenic factors in osteoblast-like UMR106 cells. Mol. Cell. Biochem. 2011, 349, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Clement-Lacroix, P.; Ormandy, C.; Lepescheux, L.; Ammann, P.; Damotte, D.; Goffin, V.; Bouchard, B.; Amling, M.; Gaillard-Kelly, M.; Binart, N.; et al. Osteoblasts are a new target for prolactin: Analysis of bone formation in prolactin receptor knockout mice. Endocrinology 1999, 140, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Piyabhan, P.; Krishnamra, N.; Limlomwongse, L. Changes in the regulation of calcium metabolism and bone calcium content during growth in the absence of endogenous prolactin and during hyperprolactinemia: A longitudinal study in male and female wistar rats. Can. J. Physiol. Pharmacol. 2000, 78, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Charoenphandhu, N. Regulation of epithelial calcium transport by prolactin: From fish to mammals. Gen. Comp. Endocrinol. 2013, 181, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Wongdee, K.; Krishnamra, N. Is prolactin the cardinal calciotropic maternal hormone? Trends Endocrinol. Metab. 2010, 21, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ormandy, C.J.; Naylor, M.; Harris, J.; Robertson, F.; Horseman, N.D.; Lindeman, G.J.; Visvader, J.; Kelly, P.A. Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Recent Prog. Horm. Res. 2003, 58, 297–323. [Google Scholar] [CrossRef] [PubMed]
- Fata, J.E.; Kong, Y.Y.; Li, J.; Sasaki, T.; Irie-Sasaki, J.; Moorehead, R.A.; Elliott, R.; Scully, S.; Voura, E.B.; Lacey, D.L.; et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 2000, 103, 41–50. [Google Scholar] [CrossRef]
- Srivastava, S.; Matsuda, M.; Hou, Z.; Bailey, J.P.; Kitazawa, R.; Herbst, M.P.; Horseman, N.D. Receptor activator of NF-κB ligand induction via JAK2 and STAT5a in mammary epithelial cells. J. Biol. Chem. 2003, 278, 46171–46178. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Soyal, S.M.; Li, J.; Ying, Y.; He, B.; DeMayo, F.J.; Lydon, J.P. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J. 2010, 24, 4408–4419. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Gallego-Ortega, D.; Ledger, A.; Schramek, D.; Joshi, P.; Szwarc, M.M.; Cho, C.; Lydon, J.P.; Khokha, R.; Penninger, J.M.; et al. Progesterone drives mammary secretory differentiation via RANKL-mediated induction of Elf5 in luminal progenitor cells. Development 2013, 140, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Pellegrini, P.; Sanz-Moreno, A.; Trinidad, E.M.; Serra-Musach, J.; Deshpande, C.; Dougall, W.C.; Pujana, M.A.; Gonzalez-Suarez, E. RANKL impairs lactogenic differentiation through inhibition of the prolactin/STAT5 pathway at midgestation. Stem Cells 2016, 34, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Thiede, M.A. The mrna encoding a parathyroid hormone-like peptide is produced in mammary tissue in response to elevations in serum prolactin. Mol. Endocrinol. 1989, 3, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Goldhar, A.S.; Vonderhaar, B.K.; Trott, J.F.; Hovey, R.C. Prolactin-induced expression of vascular endothelial growth factor via egr-1. Mol. Cell. Endocrinol. 2005, 232, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Flick, M.B.; Rodov, S.; Carter, D.; Kacinski, B.M. Expression of CSF-I and CSF-I receptor by normal lactating mammary epithelial cells. J. Soc. Gynecol. Investig. 1998, 5, 94–101. [Google Scholar] [CrossRef]
- VanHouten, J.; Dann, P.; McGeoch, G.; Brown, E.M.; Krapcho, K.; Neville, M.; Wysolmerski, J.J. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J. Clin. Investig. 2004, 113, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, H.; Barresi, C.; Ghannadan, M.; Gruber, F.; Mildner, M.; Fodinger, D.; Tschachler, E. Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J. 2007, 21, 3994–4004. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E. The role of csf-1 in normal physiology of mammary gland and breast cancer: An update. Exp. Biol. Med. 2004, 229, 1–11. [Google Scholar]
- Hui, M.; Cazet, A.; Nair, R.; Watkins, D.N.; O'Toole, S.A.; Swarbrick, A. The hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy. Breast Cancer Res. 2013, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Singh, S.; Cherukuri, P.; Li, H.; Yuan, Z.; Ellisen, L.W.; Wang, B.; Robbins, D.; DiRenzo, J. Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells. Stem Cells 2008, 26, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, Q.; Moraes, R.C.; Lewis, M.T.; Hamel, P.A. Activation of erk by sonic hedgehog independent of canonical hedgehog signalling. Int. J. Biochem. Cell Biol. 2010, 42, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.M.; Liu, B.Y.; Tlsty, T.D.; Pazour, G.J. Primary cilia regulate branching morphogenesis during mammary gland development. Curr. Biol. 2010, 20, 731–737. [Google Scholar] [CrossRef] [PubMed]



| Factor | Full Name | Target Cell | Reference |
|---|---|---|---|
| 5HT | Serotonin | OB | [69] |
| ADAMTS1 | A disintegrin and metalloproteinase with Thrombospondin motifs | OB | [70] |
| AREG | Amphiregulin | OB | [70] |
| CCL2/CCN2 | Chemokine (C–C motif) ligand 2 | stromal | [71] |
| CCN3 | Cysteine-rich protein 61, connective tissue growth factor and nephroblastoma overexpressed | OB | [72] |
| CTGF | Connective tissue growth factor | – | [73] |
| DKK-1 | Dickkopf-1 | – | [74] |
| M-CSF | Macrophage-colony stimulating factor | OC | [75] |
| GM-CSF | Granulocyte macrophage-colony stimulating factor | OC | [76] |
| HB-EGF | Heparin-binding epidermal growth factor | OB | [70] |
| IGF-1 | Insulin-like growth factor | – | [77] |
| IL-6 | Interleukin-6 | OB, OC | [57] |
| IL-8 | Interleukin-8 | OB, OC | [58,78] |
| IL-11 | Interleukin-11 | OB, OC | [79] |
| Jagged-1 | Jagged-1 | OB, OC | [80] |
| MMP-1 | Matrix metalloproteinase-1 | OC | [70,81] |
| MMP-9 | Matrix metalloproteinase-9 | – | [82] |
| OSM | Oncostatin-M | OC | [83] |
| – | Oxygen-derived free radical | OC | [84] |
| PDGF | Platelet-derived growth factor | – | [85] |
| PRDX4 | Peroxiredoxin-4 | OC | [86] |
| PTHrP | Parathyroid hormone-related protein | – | [55] |
| RANKL | Receptor activator of nuclear factor-κB ligand | OC | [62,63] |
| Sema4D | Semaforin-4D | OB | [87] |
| SHH | Sonic hedgehog | OB, OC | [88,89] |
| TGF-α | Transforming growth factor-α | OB | [70] |
| TGF-β | Transforming growth factor-β | – | [90] |
| VCAM1 | Vascular cell adhesion molecule-1 | OC | [91] |
| VEGF | Vascular endothelial growth factor | OC | [64] |
| Factor | Full Name |
|---|---|
| PTHrP | Parathyroid hormone-related protein |
| VEGF | Vascular endothelial growth factor |
| M-CSF | Macrophage colony stimulating factor |
| RANKL | receptor activator of nuclear factor-κB |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shemanko, C.S.; Cong, Y.; Forsyth, A. What Is Breast in the Bone? Int. J. Mol. Sci. 2016, 17, 1764. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101764
Shemanko CS, Cong Y, Forsyth A. What Is Breast in the Bone? International Journal of Molecular Sciences. 2016; 17(10):1764. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101764
Chicago/Turabian StyleShemanko, Carrie S., Yingying Cong, and Amanda Forsyth. 2016. "What Is Breast in the Bone?" International Journal of Molecular Sciences 17, no. 10: 1764. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17101764