Impact of Microbes on the Pathogenesis of Primary Biliary Cirrhosis (PBC) and Primary Sclerosing Cholangitis (PSC)
Abstract
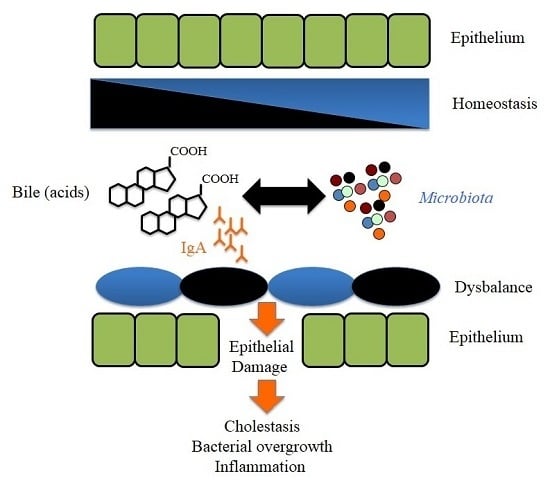
:1. Introduction
2. Mutual Interactions between the Bile and the Intestinal Microbiota
3. Association of Distinct Bacteria with Primary Sclerosing Cholangitis (PSC) and Primary Biliary Cirrhosis (PBC)
4. Changes in the Composition of the Microbiota in PBC and PSC Patients
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. Bile acids: The good, the bad, and the ugly. News Physiol. Sci. 1999, 14, 24–29. [Google Scholar] [PubMed]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. Biliary secretion and excretion in health and disease: Current concepts. Ann. Hepatol. 2007, 6, 15–27. [Google Scholar] [PubMed]
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.; Luedde, T.; Sellge, G. Biliary mucosal barrier and microbiome. Viszeralmedizin 2015, 31, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Yamamoto, K. Role of gut microbiota in liver diseases. Hepatol. Res. 2013, 43, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Gerard, P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, O.; Medici, A.; Poli, S. Biotransformations on steroid nucleus of bile acids. Steroids 1997, 62, 564–577. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Arai, H.; Nakamura, Y.; Fukiya, S.; Wada, M.; Yokota, A. Contribution of the 7β-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J. Lipid Res. 2013, 54, 3062–3069. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Shanahan, F.; Hill, C.; Gahan, C.G. Bacterial bile salt hydrolase in host metabolism: Potential for influencing gastrointestinal microbe-host crosstalk. Gut Microbes 2014, 5, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Front. Microbiol. 2014, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Zeng, X.; Lin, J. Discovery of bile salt hydrolase inhibitors using an efficient high-throughput screening system. PLoS ONE 2014, 9, e85344. [Google Scholar] [CrossRef] [PubMed]
- Dambekodi, P.C.; Gilliland, S.E. Incorporation of cholesterol into the cellular membrane of Bifidobacterium longum. J. Dairy Sci. 1998, 81, 1818–1824. [Google Scholar] [CrossRef]
- Taranto, M.P.; Fernandez Murga, M.L.; Lorca, G.; de Valdez, G.F. Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri. J. Appl. Microbiol. 2003, 95, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Farkkila, M.; Vuoristo, M.; Miettinen, T.A. Metabolism of cholesterol and low- and high-density lipoproteins in primary biliary cirrhosis: Cholesterol absorption and synthesis related to lipoprotein levels and their kinetics. Hepatology 1995, 21, 89–95. [Google Scholar] [PubMed]
- Del Puppo, M.; Galli Kienle, M.; Crosignani, A.; Petroni, M.L.; Amati, B.; Zuin, M.; Podda, M. Cholesterol metabolism in primary biliary cirrhosis during simvastatin and UDCA administration. J. Lipid Res. 2001, 42, 437–441. [Google Scholar] [PubMed]
- Kowdley, K.V. Lipids and lipid-activated vitamins in chronic cholestatic diseases. Clin. Liver Dis. 1998, 2, 373–389. [Google Scholar] [CrossRef]
- Vierling, J.M. Animal models for primary sclerosing cholangitis. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Fuchsbichler, A.; Wagner, M.; Zollner, G.; Kaser, A.; Tilg, H.; Krause, R.; Lammert, F.; Langner, C.; Zatloukal, K.; et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2004, 127, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Degirolamo, C.; Rainaldi, S.; Bovenga, F.; Murzilli, S.; Moschetta, A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014, 7, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Eckmann, L. How bile acids confer gut mucosal protection against bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 4333–4334. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.E.; Donaldson, J.R. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J. Med. Microbiol. 2009, 58, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Weingarden, A.R.; Chen, C.; Bobr, A.; Yao, D.; Lu, Y.; Nelson, V.M.; Sadowsky, M.J.; Khoruts, A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G310–G319. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Steinbruckner, B.; Brinkmann, F.E.; Ditzen, A.K.; Schwacha, H.; Aponte, J.J.; Pelz, K.; Kist, M.; Blum, H.E. Small intestinal bacterial overgrowth in patients with cirrhosis: Prevalence and relation with spontaneous bacterial peritonitis. Am. J. Gastroenterol. 2001, 96, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Zuniga, V.; Bartoli, R.; Planas, R.; Hofmann, A.F.; Vinado, B.; Hagey, L.R.; Hernandez, J.M.; Mane, J.; Alvarez, M.A.; Ausina, V.; et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology 2003, 37, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Slocum, M.M.; Sittig, K.M.; Specian, R.D.; Deitch, E.A. Absence of intestinal bile promotes bacterial translocation. Am. Surg. 1992, 58, 305–310. [Google Scholar] [PubMed]
- Ding, J.W.; Andersson, R.; Soltesz, V.; Willen, R.; Bengmark, S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur. Surg. Res. 1993, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.J.; Filburn, B.; Floch, M. Bile acid inhibition of intestinal anaerobic organisms. Am. J. Clin. Nutr. 1975, 28, 119–125. [Google Scholar] [PubMed]
- Sung, J.Y.; Shaffer, E.A.; Costerton, J.W. Antibacterial activity of bile salts against common biliary pathogens. Effects of hydrophobicity of the molecule and in the presence of phospholipids. Dig. Dis. Sci. 1993, 38, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Nieman, C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol. Rev. 1954, 18, 147–163. [Google Scholar] [PubMed]
- Zheng, C.J.; Yoo, J.S.; Lee, T.G.; Cho, H.Y.; Kim, Y.H.; Kim, W.G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Aerobic and anaerobic microbiology of biliary tract disease. J. Clin. Microbiol. 1989, 27, 2373–2375. [Google Scholar] [PubMed]
- Carpenter, H.A. Bacterial and parasitic cholangitis. Mayo Clin. Proc. 1998, 73, 473–478. [Google Scholar] [CrossRef]
- Ganzle, M.G.; Hertel, C.; van der Vossen, J.M.; Hammes, W.P. Effect of bacteriocin-producing lactobacilli on the survival of Escherichia coli and Listeria in a dynamic model of the stomach and the small intestine. Int. J. Food Microbiol. 1999, 48, 21–35. [Google Scholar] [CrossRef]
- Fox, J.G.; Yan, L.L.; Dewhirst, F.E.; Paster, B.J.; Shames, B.; Murphy, J.C.; Hayward, A.; Belcher, J.C.; Mendes, E.N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J. Clin. Microbiol. 1995, 33, 445–454. [Google Scholar] [PubMed]
- Hirai, Y. The interaction of bile acids and Helicobacter pylori. J. Gastroenterol. 1999, 34, 653–654. [Google Scholar] [PubMed]
- Daniels, J.A.; Torbenson, M.; Anders, R.A.; Boitnott, J.K. Immunostaining of plasma cells in primary biliary cirrhosis. Am. J. Clin. Pathol. 2009, 131, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Feizi, T. Immunoglobulins in chronic liver disease. Gut 1968, 9, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.K.; Revetta, F.; Koehler, E.; Washington, M.K. Diagnostic utility of IgG and IgM immunohistochemistry in autoimmune liver disease. World J. Gastroenterol. 2010, 16, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.K.; Rosenstein, I.J.; Epstein, O.; Hamilton-Miller, J.M.; Brumfitt, W.; Sherlock, S. Bacteriuria and primary biliary cirrhosis. Gut 1984, 25, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.; Valle, F.; Hamilton-Miller, J.M.; Brumfitt, W.; Baum, H.; Burroughs, A.K. M2 mitochondrial antibodies and urinary rough mutant bacteria in patients with primary biliary cirrhosis and in patients with recurrent bacteriuria. J. Hepatol. 1993, 17, 408–414. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Baum, H.; Butler, P.; Rigopoulou, E.I.; Davies, E.T.; Ma, Y.; Burroughs, A.K.; Vergani, D. Association between the primary biliary cirrhosis specific anti-sp100 antibodies and recurrent urinary tract infection. Dig. Liver Dis. 2003, 35, 801–805. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Selmi, C.; Worman, H.J.; Gold, E.B.; Watnik, M.; Utts, J.; Lindor, K.D.; Kaplan, M.M.; Vierling, J.M.; the USA PBC Epidemiology Group. Risk factors and comorbidities in primary biliary cirrhosis: A controlled interview-based study of 1032 patients. Hepatology 2005, 42, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.K.; Butler, P.; Sternberg, M.J.; Baum, H. Molecular mimicry in liver disease. Nature 1992, 358, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Chretien, Y.; Chazouilleres, O.; Poupon, R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J. Hepatol. 2010, 53, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.E.; Talwalkar, J.A.; Lazaridis, K.N.; Gores, G.J.; Lindor, K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013, 145, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Sandborn, W.J.; Lindor, K.D.; Larusso, N.F. Interactions between chronic liver disease and inflammatory bowel disease. Inflamm. Bowel Dis. 1997, 3, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, A.S.; Petrovic, L.M.; Kim, W.R.; Angulo, P.; Lloyd, R.V.; Lindor, K.D. Primary biliary cirrhosis: An infectious disease caused by Chlamydia pneumoniae? J. Hepatol. 2004, 40, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Hopf, U.; Moller, B.; Stemerowicz, R.; Lobeck, H.; Rodloff, A.; Freudenberg, M.; Galanos, C.; Huhn, D. Relation between Escherichia coli R(rough)-forms in gut, lipid A in liver, and primary biliary cirrhosis. Lancet 1989, 2, 1419–1422. [Google Scholar] [CrossRef]
- Smyk, D.S.; Rigopoulou, E.I.; Bogdanos, D.P. Potential Roles for infectious agents in the pathophysiology of primary biliary cirrhosis: What’s new? Curr. Infect. Dis. Rep. 2013, 15, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.O.; Taneera, J.; Castedal, M.; Glatz, E.; Olsson, R.; Wadstrom, T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J. Clin. Microbiol. 2000, 38, 1072–1076. [Google Scholar] [PubMed]
- Nilsson, H.O.; Taneera, J.; Castedal, M.; Wadstrom, T.; Olsson, R. Infectious agents and primary biliary cirrhosis. J. Hepatol. 2000, 33, 342–343. [Google Scholar] [CrossRef]
- Xu, L.; Shen, Z.; Guo, L.; Fodera, B.; Keogh, A.; Joplin, R.; O’Donnell, B.; Aitken, J.; Carman, W.; Neuberger, J.; et al. Does a β retrovirus infection trigger primary biliary cirrhosis? Proc. Natl. Acad. Sci. USA 2003, 100, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Bjornsson, E.; Backman, L.; Friman, S.; Hockerstedt, K.; Kaijser, B.; Olausson, M. Bile duct bacterial isolates in primary sclerosing cholangitis: A study of explanted livers. J. Hepatol. 1998, 28, 426–432. [Google Scholar] [CrossRef]
- Pollheimer, M.J.; Halilbasic, E.; Fickert, P.; Trauner, M. Pathogenesis of primary sclerosing cholangitis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Fussey, S.P.; Ali, S.T.; Guest, J.R.; James, O.F.; Bassendine, M.F.; Yeaman, S.J. Reactivity of primary biliary cirrhosis sera with Escherichia coli dihydrolipoamide acetyltransferase (E2p): Characterization of the main immunogenic region. Proc. Natl. Acad. Sci. USA 1990, 87, 3987–3991. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Balkwill, D.L.; Invernizzi, P.; Ansari, A.A.; Coppel, R.L.; Podda, M.; Leung, P.S.; Kenny, T.P.; van de Water, J.; Nantz, M.H.; et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology 2003, 38, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Bogdanos, D.P.; Baum, H.; Grasso, A.; Okamoto, M.; Butler, P.; Ma, Y.; Rigopoulou, E.; Montalto, P.; Davies, E.T.; Burroughs, A.K.; et al. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J. Hepatol. 2004, 40, 31–39. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Baum, H.; Okamoto, M.; Montalto, P.; Sharma, U.C.; Rigopoulou, E.I.; Vlachogiannakos, J.; Ma, Y.; Burroughs, A.K.; Vergani, D. Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology 2005, 42, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Bogdanos, D.P.; Pares, A.; Baum, H.; Caballeria, L.; Rigopoulou, E.I.; Ma, Y.; Burroughs, A.K.; Rodes, J.; Vergani, D. Disease-specific cross-reactivity between mimicking peptides of heat shock protein of Mycobacterium gordonae and dominant epitope of E2 subunit of pyruvate dehydrogenase is common in Spanish but not British patients with primary biliary cirrhosis. J. Autoimmun. 2004, 22, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.A.; Selmi, C.; Kenny, T.P.; Leung, P.S.; Balkwill, D.L.; Ansari, A.A.; Coppel, R.L.; Gershwin, M.E. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J. Autoimmun. 2005, 24, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.M.; Gershwin, M.E. Primary biliary cirrhosis. N. Engl. J. Med. 2005, 353, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Baum, H.; Bogdanos, D.P.; Vergani, D. Antibodies to Clp protease in primary biliary cirrhosis: Possible role of a mimicking T-cell epitope. J. Hepatol. 2001, 34, 785–787. [Google Scholar] [CrossRef]
- Barbeau, J.; Tanguay, R.; Faucher, E.; Avezard, C.; Trudel, L.; Cote, L.; Prevost, A.P. Multiparametric analysis of waterline contamination in dental units. Appl. Environ. Microbiol. 1996, 62, 3954–3959. [Google Scholar] [PubMed]
- Cavicchioli, R.; Fegatella, F.; Ostrowski, M.; Eguchi, M.; Gottschal, J. Sphingomonads from marine environments. J. Ind. Microbiol. Biotechnol. 1999, 23, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Brodie, E.L.; DeSantis, T.Z.; Parker, J.P.; Zubietta, I.X.; Piceno, Y.M.; Andersen, G.L. Urban aerosols harbor diverse and dynamic bacterial populations. Proc. Natl. Acad. Sci. USA 2007, 104, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Mattner, J.; Savage, P.B.; Leung, P.; Oertelt, S.S.; Wang, V.; Trivedi, O.; Scanlon, S.T.; Pendem, K.; Teyton, L.; Hart, J.; et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe 2008, 3, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.P.; Fusakio, M.E.; Rainbow, D.B.; Moule, C.; Fraser, H.I.; Clark, J.; Todd, J.A.; Peterson, L.B.; Savage, P.B.; Wills-Karp, M.; et al. Identification of Cd101 as a susceptibility gene for Novosphingobium aromaticivorans-induced liver autoimmunity. J. Immunol. 2011, 187, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Mattner, J. Natural killer T (NKT) cells in autoimmune hepatitis. Curr. Opin. Immunol. 2013, 25, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, K.L.; Sung, J.; Kim, E.; Cerniglia, C.E. Physiological and genetic comparison of two aromatic hydrocarbon-degrading Sphingomonas strains. Mol. Cells 2000, 10, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Fredrickson, J.K.; Balkwill, D.L. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas strains isolated from the terrestrial subsurface. J. Ind. Microbiol. Biotechnol. 2001, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pinyakong, O.; Habe, H.; Omori, T. The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons (PAHs). J. Gen. Appl. Microbiol. 2003, 49, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Carbone, M.; Lleo, A.; Invernizzi, P. Geoepidemiology, genetic and environmental risk factors for PBC. Dig. Dis. 2015, 2 (Suppl. 33), 94–101. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.V.; Mitchison, H.C.; Palmer, J.M.; Jones, D.E.; Bassendine, M.F.; James, O.F. Natural history of early primary biliary cirrhosis. Lancet 1996, 348, 1399–1402. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Adams, D.H. Mucosal immunity in liver autoimmunity: A comprehensive review. J. Autoimmun. 2013, 46, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Katt, J.; Schwinge, D.; Schoknecht, T.; Quaas, A.; Sobottka, I.; Burandt, E.; Becker, C.; Neurath, M.F.; Lohse, A.W.; Herkel, J.; et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013, 58, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Folseraas, T.; Melum, E.; Rausch, P.; Juran, B.D.; Ellinghaus, E.; Shiryaev, A.; Laerdahl, J.K.; Ellinghaus, D.; Schramm, C.; Weismuller, T.J.; et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 2012, 57, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiramatsu, K.; Harada, K.; Tsuneyama, K.; Sasaki, M.; Fujita, S.; Hashimoto, T.; Kaneko, S.; Kobayashi, K.; Nakanuma, Y. Amplification and sequence analysis of partial bacterial 16S ribosomal RNA gene in gallbladder bile from patients with primary biliary cirrhosis. J. Hepatol. 2000, 33, 9–18. [Google Scholar] [CrossRef]
- Jimenez, E.; Sanchez, B.; Farina, A.; Margolles, A.; Rodriguez, J.M. Characterization of the bile and gall bladder microbiota of healthy pigs. Microbiol. Open 2014, 3, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Packey, C.D.; Sartor, R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009, 22, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.; Kelly, D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int. J. Med. Microbiol. 2010, 300, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Talwalkar, J.A.; Lindor, K.D. Role of the microbiota and antibiotics in primary sclerosing cholangitis. BioMed Res. Int. 2013, 2013, 389537. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, S.N.; Keku, J.; Clark, R.L.; Schwab, J.H.; Sartor, R.B. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology 1991, 13, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, S.N.; Okoruwa, E.E.; Keku, J.; Schwab, J.H.; Sartor, R.B. Degradation of endogenous bacterial cell wall polymers by the muralytic enzyme mutanolysin prevents hepatobiliary injury in genetically susceptible rats with experimental intestinal bacterial overgrowth. J. Clin. Investig. 1992, 90, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; Boonstra, K.; D’Haens, G.R.; Heilig, H.G.; Zoetendal, E.G.; de Vos, W.M.; Ponsioen, C.Y. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J. Crohns Colitis 2015, 9, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Sergeant, M.; Kay, G.; Iqbal, T.; Chan, J.; Constantinidou, C.; Trivedi, P.; Ferguson, J.; Adams, D.H.; Pallen, M.; et al. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut 2016. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; van Assche, G.; van der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Rupp, C.; Bode, K.A.; Chahoud, F.; Wannhoff, A.; Friedrich, K.; Weiss, K.H.; Sauer, P.; Stremmel, W.; Gotthardt, D.N. Risk factors and outcome in patients with primary sclerosing cholangitis with persistent biliary candidiasis. BMC Infect. Dis. 2014, 14, 562. [Google Scholar] [CrossRef] [PubMed]
- Rausch, P.; Rehman, A.; Kunzel, S.; Hasler, R.; Ott, S.J.; Schreiber, S.; Rosenstiel, P.; Franke, A.; Baines, J.F. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl. Acad. Sci. USA 2011, 108, 19030–19035. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Maurice, C.F.; Kinnebrew, M.A.; Abt, M.C.; Schenten, D.; Golovkina, T.V.; Bogatyrev, S.R.; Ismagilov, R.F.; Pamer, E.G.; Turnbaugh, P.J.; et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 2014, 514, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Terjung, B.; Sohne, J.; Lechtenberg, B.; Gottwein, J.; Muennich, M.; Herzog, V.; Mahler, M.; Sauerbruch, T.; Spengler, U. p-ANCAs in autoimmune liver disorders recognise human β-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut 2010, 59, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Karrar, A.; Broome, U.; Sodergren, T.; Jaksch, M.; Bergquist, A.; Bjornstedt, M.; Sumitran-Holgersson, S. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology 2007, 132, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; O’Hara, S.P.; Trussoni, C.E.; Tietz, P.S.; Splinter, P.L.; Mounajjed, T.; Hagey, L.R.; LaRusso, N.F. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 2016, 63, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.L.; Cox, K.M. Oral vancomycin: Treatment of primary sclerosing cholangitis in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Farkkila, M.; Karvonen, A.L.; Nurmi, H.; Nuutinen, H.; Taavitsainen, M.; Pikkarainen, P.; Karkkainen, P. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: A randomized placebo-controlled trial. Hepatology 2004, 40, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Davies, Y.K.; Cox, K.M.; Abdullah, B.A.; Safta, A.; Terry, A.B.; Cox, K.L. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: An immunomodulating antibiotic. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.G.; Torok, N.J.; Gossard, A.A.; Keach, J.C.; Jorgensen, R.A.; Petz, J.L.; Lindor, K.D. Minocycline in the treatment of patients with primary sclerosing cholangitis: Results of a pilot study. Am. J. Gastroenterol. 2009, 104, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, D.A.; Lindor, K.D. Antibiotics for the treatment of primary sclerosing cholangitis. Am. J. Ther. 2011, 18, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Weeding, E.; Jorgensen, R.A.; Petz, J.L.; Keach, J.C.; Talwalkar, J.A.; Lindor, K.D. Randomised clinical trial: Vancomycin or metronidazole in patients with primary sclerosing cholangitis—A pilot study. Aliment. Pharmacol. Ther. 2013, 37, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Goode, E.C.; Rushbrook, S.M. A review of the medical treatment of primary sclerosing cholangitis in the 21st century. Ther. Adv. Chronic Dis. 2016, 7, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. How antibiotics can make us sick: The less obvious adverse effects of antimicrobial chemotherapy. Lancet Infect. Dis. 2004, 4, 611–619. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [PubMed]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Mogg, G.A.; Keighley, M.R.; Burdon, D.W.; Alexander-Williams, J.; Youngs, D.; Johnson, M.; Bentley, S.; George, R.H. Antibiotic-associated colitis—A review of 66 cases. Br. J. Surg. 1979, 66, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Bartlett, J.G. Antibiotic-associated diarrhea. N. Engl. J. Med. 2002, 346, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, D.A.; Huse, S.M.; Morrison, H.G.; Schmidt, T.M.; Sogin, M.L.; Young, V.B. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 2009, 77, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Hoffmann, C.; Abt, M.C.; Du, Y.; Kobuley, D.; Kirn, T.J.; Bushman, F.D.; Artis, D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010, 3, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; van den Brink, M.R.; Kamboj, M.; et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Svanstrom, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Siracusa, M.C.; Abt, M.C.; Kim, B.S.; Kobuley, D.; Kubo, M.; Kambayashi, T.; Larosa, D.F.; Renner, E.D.; Orange, J.S.; et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 2012, 18, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Gold, M.J.; Hartmann, M.; Willing, B.P.; Thorson, L.; Wlodarska, M.; Gill, N.; Blanchet, M.R.; Mohn, W.W.; McNagny, K.M.; et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012, 13, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.L.; Morgun, A.; Shulzhenko, N. Bridging immunity and lipid metabolism by gut microbiota. J. Allergy Clin. Immunol. 2013, 132, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Weingarden, A.R.; Chen, C.; Zhang, N.; Graiziger, C.T.; Dosa, P.I.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Sadowsky, M.J.; Khoruts, A. Ursodeoxycholic acid inhibits clostridium difficile spore germination and vegetative growth, and prevents the recurrence of ileal pouchitis associated with the infection. J. Clin. Gastroenterol. 2016, 50, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Fukiya, S.; Islam, K.B.; Ooka, T.; Ogura, Y.; Hayashi, T.; Hagio, M.; Ishizuka, S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012, 3, 455–459. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattner, J. Impact of Microbes on the Pathogenesis of Primary Biliary Cirrhosis (PBC) and Primary Sclerosing Cholangitis (PSC). Int. J. Mol. Sci. 2016, 17, 1864. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111864
Mattner J. Impact of Microbes on the Pathogenesis of Primary Biliary Cirrhosis (PBC) and Primary Sclerosing Cholangitis (PSC). International Journal of Molecular Sciences. 2016; 17(11):1864. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111864
Chicago/Turabian StyleMattner, Jochen. 2016. "Impact of Microbes on the Pathogenesis of Primary Biliary Cirrhosis (PBC) and Primary Sclerosing Cholangitis (PSC)" International Journal of Molecular Sciences 17, no. 11: 1864. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111864