Varietal Dependence of GLVs Accumulation and LOX-HPL Pathway Gene Expression in Four Vitis vinifera Wine Grapes
Abstract
:1. Introduction
2. Results and Discussion
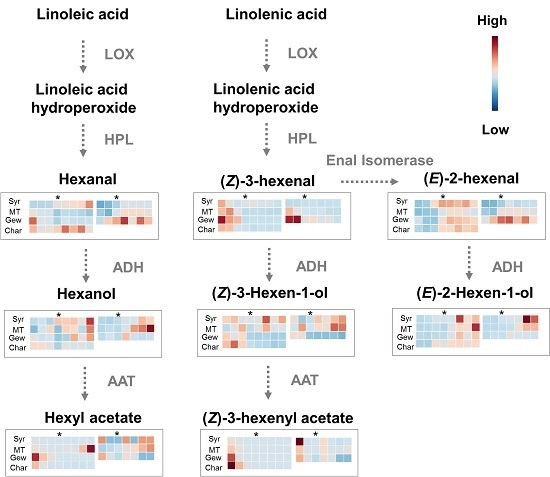
2.1. Green Leaf Volatiles (GLVs) Profiling during Berry Development of Four Wine Grape Varieties
2.2. Varietal Effect on the Concentrations of GLVs in Grape Berries by Multivariate Analysis
2.3. Comparison of Expression of Lipoxygenase-Hydroperoxides lyase (LOX-HPL) Pathway Genes in Different Grape Varieties during Berry Development
3. Materials and Methods
3.1. Sample Collection
3.2. Extraction and GC-MS Analysis of Volatile Compounds
3.3. Total RNA Extraction, Purification, cDNA Synthesis and Real-Time qPCR Assay
3.4. Statistical Analysis
3.5. Reagents and Standards
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Gigot, C.; Ongena, M.; Fauconnier, M.L.; Wathelet, J.P.; du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar]
- Gomez, E.; Martinez, A.; Laencina, J. Changes in volatile compounds during maturation of some grape varieties. J. Sci. Food Agric. 1995, 67, 229–233. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Bottcher, C.; Davies, C. Various influences of harvest date and fruit sugar content on different wine flavor and aroma compounds. Am. J. Enol. Viticult. 2014, 65, 341–353. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Aust. J. Grape Wine Res. 2010, 16, 337–348. [Google Scholar] [CrossRef]
- Podolyan, A.; White, J.; Jordan, B.; Winefield, C. Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13-lipoxygenases expressed in grape berries of Sauvignon Blanc. Funct. Plant Biol. 2010, 37, 767–784. [Google Scholar] [CrossRef]
- Zhu, B.-Q.; Xu, X.-Q.; Wu, Y.-W.; Duan, C.-Q.; Pan, Q.-H. Isolation and characterization of two hydroperoxide lyase genes from grape berries. Mol. Biol. Rep. 2012, 39, 7443–7455. [Google Scholar] [CrossRef] [PubMed]
- Tesnière, C.; Verriès, C. Molecular cloning and expression of cDNAs encoding alcohol dehydrogenases from Vitis vinifera L. during berry development. Plant Sci. 2000, 157, 77–88. [Google Scholar] [CrossRef]
- Shiojiri, K.; Kishimoto, K.; Ozawa, R.; Kugimiya, S.; Urashimo, S.; Arimura, G.; Horiuchi, J.; Nishioka, T.; Matsui, K.; Takabayashi, J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Pare, P.W. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 2002, 61, 545–554. [Google Scholar] [CrossRef]
- Tesniere, C.; Torregrosa, L.; Pradal, M.; Souquet, J.M.; Gilles, C.; Dos Santos, K.; Chatelet, P.; Gunata, Z. Effects of genetic manipulation of alcohol dehydrogenase levels on the response to stress and the synthesis of secondary metabolites in grapevine leaves. J. Exp. Bot. 2006, 57, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Viticult. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Mateo, J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. Cv. Melon B. as precursors of odorants in Muscadet wines. J. Chromatogr. A 2001, 936, 145–157. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Antalick, G.; Suklje, K.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M. Influence of grape composition on red wine ester profile: Comparison between Cabernet Sauvignon and Shiraz cultivars from australian warm climate. J. Agric. Food Chem. 2015, 63, 4664–4672. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Q.; Cheng, G.; Duan, L.-L.; Jiang, R.; Pan, Q.-H.; Duan, C.-Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.; McCarthy, M.; Ford, C.M.; Dokoozlian, N. Seasonal and regional variation of green aroma compounds in commercial vineyards of Vitis vinifera L. Merlot in California. Am. J. Enol. Viticult. 2013, 64, 430–436. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N.K. Effect of increased irrigation and additional nitrogen fertilisation on the concentration of green aroma compounds in Vitis vinifera L. Merlot fruit and wine. Aust. J. Grape Wine Res. 2014, 20, 80–90. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Orellana, A.; Mercenaro, L.; Shackel, K.A.; Willits, N.; Matthews, M.A. Responses of fruit uniformity to deficit irrigation and cluster thinning in commercial winegrape production. Am. J. Enol. Viticult. 2014, 65, 354–362. [Google Scholar] [CrossRef]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; McCarthy, M.; Ford, C.M.; Dokoozlian, N. Effect of winter rainfall on yield components and fruit green aromas of Vitis vinifera L. Cv. Merlot in California. Aust. J. Grape Wine Res. 2014, 20, 100–110. [Google Scholar] [CrossRef]
- Pedneault, K.; Dorais, M.; Angers, P. Flavor of cold-hardy grapes: Impact of berry maturity and environmental conditions. J. Agric. Food Chem. 2013, 61, 10418–10438. [Google Scholar] [CrossRef] [PubMed]
- García, E.; Chacón, J.; Martínez, J.; Izquierdo, P. Changes in volatile compounds during ripening in grapes of Airén, Macabeo and Chardonnay white varieties grown in La Mancha region (Spain). Food Sci. Technol. Int. 2003, 9, 33–41. [Google Scholar] [CrossRef]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. Metaboanalyst 2.0—A comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef] [PubMed]
- Ebang-Oke, J.P.; Billerbeck, G.M.d.; Ambid, C.; Le Quéré, J.L.; Étiévant, P.X. Temporal expression of the Lis gene from Vitis vinifera L., cv. Muscat de Frontignan. In Flavour Research at the Dawn of the Twenty-First Century, Proceedings of the 10th Weurman Flavour Research Symposium, Beaune, France, 25–28 June 2002; le Quere, J.L., Etievant, P.X., Eds.; Lavoisier: London, UK, 2003; pp. 321–325. [Google Scholar]
- Katarína, F.; Katarína, M.; Katarína, Ď.; Ivan, Š.; Fedor, M. Influence of yeast strain on aromatic profile of Gewürztraminer wine. LWT-Food Sci. Technol. 2014, 59, 256–262. [Google Scholar] [CrossRef]
- Ong, P.K.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis Sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs. terroir effects. Food Chem. 2014, 152, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.E.; Gaudillere, J.-P.; Leeuwen, C.V.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR metabolite fingerprints of grape berry: Comparison of vintage and soil effects in Bordeaux grapevine growing areas. Anal. Chim. Acta 2006, 563, 346–352. [Google Scholar] [CrossRef]
- Ul Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Merillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-Q.; He, F.; Zhu, B.-Q.; Lan, Y.-B.; Pan, Q.-H.; Li, C.-Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef] [PubMed]
- Marshall, O.J. Perlprimer: Cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 2004, 20, 2471–2472. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2012. Available online: http://www.R-project.Org (accessed on 10 July 2014).




| Genes | Hexanal | (E)-2-Hexenal | (Z)-3-Hexenal | 1-Hexanol | (Z)-3-Hexen-1-ol | (E)-2-Hexen-1-ol | Hexyl Acetate | (Z)-3-Hexenyl Acetate | Sum of C6 Aldehydes | Sum of C6 Alcohols | Sum of C6 Esters |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chardonnay | |||||||||||
| LOXO | −0.984 | 0.811 | 0.728 | 0.962 | −0.757 | −0.865 | 0.719 | ||||
| ADH1 | −0.746 | −0.868 | |||||||||
| ADH2 | 0.807 | −0.761 | |||||||||
| AAT | 0.765 | 0.741 | |||||||||
| Gewürztraminer | |||||||||||
| LOXA | 0.771 | ||||||||||
| LOXO | −0.550 | 0.581 | |||||||||
| ADH1 | −0.659 | 0.704 | |||||||||
| ADH2 | 0.856 | −0.653 | −0.667 | ||||||||
| AAT | −0.661 | −0.639 | |||||||||
| Muscat Tchervine | |||||||||||
| LOXO | 0.761 | 0.774 | 0.582 | 0.592 | |||||||
| HPL1 | 0.639 | −0.653 | |||||||||
| AAT | 0.629 | 0.568 | |||||||||
| Syrah | |||||||||||
| LOXA | −0.574 | −0.662 | |||||||||
| HPL1 | 0.541 | ||||||||||
| ADH2 | −0.550 | −0.558 | |||||||||
| ADH3 | −0.575 | −0.638 | |||||||||
| AAT | 0.639 | 0.654 | 0.673 | 0.796 | |||||||
| DAF | Muscat Tchervine | Gewürztraminer | Syrah | Chardonnay | ||||
|---|---|---|---|---|---|---|---|---|
| 2010 | Brix | pH | Brix | pH | Brix | pH | Brix | pH |
| 31 | 4.4 | 2.5 | 3.8 | 2.6 | 4.0 | 2.5 | 4.0 | 2.6 |
| 43 | 5.7 | 2.5 | 4.8 | 2.7 | 4.8 | 2.5 | 3.9 | 2.7 |
| 59 | 8.3 | 2.6 | 8.2 | 2.9 | 5.1 | 2.7 | 10.6 | 2.8 |
| 73 | 19.5 | 3.0 | 15.9 | 3.0 | 13.8 | 2.9 | 18.5 | 3.2 |
| 87 | 17.5 | 2.9 | 18.8 | 3.0 | 16.5 | 3.2 | 15.8 | 3.1 |
| 101 | 20.4 | 3.0 | 22.5 | 3.2 | 19.3 | 3.3 | 20.9 | 3.4 |
| 106 | 28.1 | 3.2 | 27.6 | 3.3 | 21.6 | 3.3 | 20.1 | 3.3 |
| 2011 | Brix | pH | Brix | pH | Brix | pH | - | - |
| 32 | 5.8 | 2.5 | 4.5 | 2.6 | 5.2 | 2.4 | - | - |
| 48 | 6.7 | 2.5 | 6.9 | 2.6 | 5.6 | 2.5 | - | - |
| 60 | 11.7 | 2.6 | 14.0 | 2.9 | 8.3 | 2.5 | - | - |
| 75 | 18.7 | 3.0 | 17.5 | 3.2 | 16.9 | 2.9 | - | - |
| 89 | 19.1 | 3.1 | 21.3 | 3.2 | 17.5 | 3.2 | - | - |
| 102 | 24.2 | 3.2 | 18.4 | 3.1 | 21.4 | 3.3 | - | - |
| 112 | 28.1 | 3.2 | 25.8 | 3.5 | 23.6 | 3.3 | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Xu, X.-Q.; Yu, K.-J.; Zhu, B.-Q.; Lan, Y.-B.; Duan, C.-Q.; Pan, Q.-H. Varietal Dependence of GLVs Accumulation and LOX-HPL Pathway Gene Expression in Four Vitis vinifera Wine Grapes. Int. J. Mol. Sci. 2016, 17, 1924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111924
Qian X, Xu X-Q, Yu K-J, Zhu B-Q, Lan Y-B, Duan C-Q, Pan Q-H. Varietal Dependence of GLVs Accumulation and LOX-HPL Pathway Gene Expression in Four Vitis vinifera Wine Grapes. International Journal of Molecular Sciences. 2016; 17(11):1924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111924
Chicago/Turabian StyleQian, Xu, Xiao-Qing Xu, Ke-Ji Yu, Bao-Qing Zhu, Yi-Bin Lan, Chang-Qing Duan, and Qiu-Hong Pan. 2016. "Varietal Dependence of GLVs Accumulation and LOX-HPL Pathway Gene Expression in Four Vitis vinifera Wine Grapes" International Journal of Molecular Sciences 17, no. 11: 1924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111924