The Coexistence of Hypertension and Ovariectomy Additively Increases Cardiac Apoptosis
Abstract
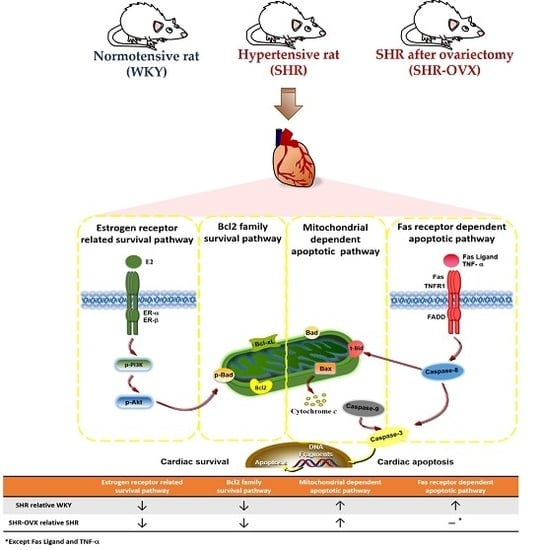
:1. Introduction
2. Results
2.1. Body Weight and Cardiac Characteristics
2.2. Cardiac Histopathological Changes
2.3. TUNEL-Positive Apoptotic Cells of Left Ventricle
2.4. Components of Cardiac Estrogen Receptors
2.5. Cardiac Bcl-2 Family Survival Pathway
2.6. Cardiac Mitochondria-Dependent Apoptotic Pathways
2.7. Cardiac Fas Receptor-Dependent Apoptotic Pathways
3. Discussion
Hypothesized and Clinical Application
4. Experimental Section
4.1. Animals
4.2. Ovariectomized Rat Model
4.3. Resting Blood Pressure and Heart Rate
4.4. Cardiac Characteristics
4.5. Hematoxylin-Eosin Staining
4.6. Masson’s Trichrome Staining
4.7. DAPI and TUNEL Staining
4.8. Western Immunoblotting
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Staessen, J.A.; Celis, H.; Fagard, R. The epidemiology of the association between hypertension and menopause. J. Hum. Hypertens. 1998, 12, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Grimaldi, T.; Origliani, G.; Fantini, G.; Coppi, F.; Modena, M.G. Menopause and cardiovascular risk. Pathophysiol. Haemost. Thromb. 2002, 32, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Fleg, J.L. Hypertension in postmenopausal women as a medical and public health problem. High Blood Press. Cardiovasc. Prev. 2003, 10, 51–55. [Google Scholar] [CrossRef]
- Moolman, J.A. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc. Res. 2006, 69, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Fabris, B.; Candido, R.; Bortoletto, M.; Toffoli, B.; Bernardi, S.; Stebel, M.; Bardelli, M.; Zentilin, L.; Giacca, M.; Carretta, R. Stimulation of cardiac apoptosis in ovariectomized hypertensive rats: Potential role of the renin-angiotensin system. J. Hypertens. 2011, 29, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Arenas, I.A.; Armstrong, S.J.; Davidge, S.T. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc. Res. 2003, 57, 388–394. [Google Scholar] [CrossRef]
- Grohe, C.; Kahlert, S.; Lobbert, K.; Stimpel, M.; Karas, R.H.; Vetter, H.; Neyses, L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997, 416, 107–112. [Google Scholar] [CrossRef]
- Patten, R.D.; Pourati, I.; Aronovitz, M.J.; Baur, J.; Celestin, F.; Chen, X.; Michael, A.; Haq, S.; Nuedling, S.; Grohe, C.; et al. 17β-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ. Res. 2004, 95, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Bishopric, N.H.; Andreka, P.; Slepak, T.; Webster, K.A. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr. Opin. Pharmacol. 2001, 1, 141–150. [Google Scholar] [CrossRef]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 2001, 26, 61–66. [Google Scholar] [CrossRef]
- Grinberg, M.; Sarig, R.; Zaltsman, Y.; Frumkin, D.; Grammatikakis, N.; Reuveny, E.; Gross, A. tBID homooligomerizes in the mitochondrial membrane to induce apoptosis. J. Biol. Chem. 2002, 277, 12237–12245. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.R.; Tappia, P.S.; Dhalla, N.S. Gender differences in apoptotic signaling in heart failure due to volume overload. Apoptosis 2010, 15, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.M.; Yang, A.L.; Kuo, C.H.; Tin, H.; Huang, C.Y.; Lee, S.D. Effects of 17β-estradiol on cardiac apoptosis in ovariectomized rats. Cell Biochem. Funct. 2010, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Bernecker, O.Y.; Huq, F.; Heist, E.K.; Podesser, B.K.; Hajjar, R.J. Apoptosis in heart failure and the senescent heart. Cardiovasc. Toxicol. 2003, 3, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Kuwahara, F.; Tokuda, K.; Imaizumi, T. Diastolic dysfunction in hypertensive hearts: Roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens. Res. 2005, 28, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Gurtl, B.; Kratky, D.; Guelly, C.; Zhang, L.; Gorkiewicz, G.; Das, S.K.; Tamilarasan, K.P.; Hoefler, G. Apoptosis and fibrosis are early features of heart failure in an animal model of metabolic cardiomyopathy. Int. J. Exp. Pathol. 2009, 90, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ares-Carrasco, S.; Picatoste, B.; Benito-Martin, A.; Zubiri, I.; Sanz, A.B.; Sanchez-Nino, M.D.; Ortiz, A.; Egido, J.; Tunon, J.; Lorenzo, O. Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, H2109–H2119. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Haider, N.; Arbustini, E.; Chandrashekhar, Y. Mechanisms of disease: Apoptosis in heart failure—Seeing hope in death. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yang, A.L.; Lin, Y.M.; Wu, F.N.; Lin, J.A.; Chan, Y.S.; Tsai, F.J.; Tsai, C.H.; Kuo, C.H.; Lee, S.D. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J. Appl. Physiol. 2012, 112, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Kuo, W.W.; Ho, Y.J.; Lin, A.C.; Tsai, C.H.; Wang, H.F.; Kuo, C.H.; Yang, A.L.; Huang, C.Y.; Hwang, J.M. Cardiac Fas-dependent and mitochondria-dependent apoptosis in ovariectomized rats. Maturitas 2008, 61, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.; Fukunaga, K. Characterization of an animal model of postmenopausal cardiac hypertrophy and novel mechanisms responsible for cardiac decompensation using ovariectomized pressure-overloaded rats. Menopause 2010, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Katholi, R.E.; Couri, D.M. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. Int. J. Hypertens. 2011, 2011, 495349. [Google Scholar] [CrossRef] [PubMed]
- Voloshenyuk, T.G.; Gardner, J.D. Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R683–R693. [Google Scholar] [CrossRef] [PubMed]
- Diez, J. Mechanisms of cardiac fibrosis in hypertension. J. Clin. Hypertens. 2007, 9, 546–550. [Google Scholar] [CrossRef]
- Mancia, G.; Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Adrenal signaling in heart failure: Something more than a distant ship’s smoke on the horizon. Hypertension 2014, 63, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Trimarco, B.; Iaccarino, G. G-protein-coupled receptor kinase 2 and hypertension: Molecular insights and pathophysiological mechanisms. High Blood Press. Cardiovasc. Prev. 2013, 20, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Communal, C.; Colucci, W.S. The control of cardiomyocyte apoptosis via the β-adrenergic signaling pathways. Arch. Mal. Coeur Vaiss. 2005, 98, 236–241. [Google Scholar] [PubMed]
- Martin, D.S.; Redetzke, R.; Vogel, E.; Mark, C.; Eyster, K.M. Effect of ovariectomy on blood pressure and venous tone in female spontaneously hypertensive rats. Am. J. Hypertens. 2008, 21, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Tatchum-Talom, R.; Eyster, K.M.; Kost, C.K., Jr.; Martin, D.S. Blood pressure and mesenteric vascular reactivity in spontaneously hypertensive rats 7 months after gonadectomy. J. Cardiovasc. Pharmacol. 2011, 57, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.K.; Coglan, M.J.; Wright, G.R.; de Voss, K.N.; Battistutta, D. Hormone therapy in women in the menopause transition. Randomised, double-blind, placebo-controlled trial of effects on body weight, blood pressure, lipoprotein levels, antithrombin III activity, and the endometrium. Med. J. Aust. 1998, 168, 216–220. [Google Scholar] [PubMed]
- Hinojosa-Laborde, C.; Craig, T.; Zheng, W.; Ji, H.; Haywood, J.R.; Sandberg, K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 2004, 44, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Weil, B.; Abarbanell, A.; Herrmann, J.; Tan, J.; Kelly, M.; Meldrum, D.R. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R972–R978. [Google Scholar] [CrossRef] [PubMed]
- Sriramula, S.; Haque, M.; Majid, D.S.; Francis, J. Involvement of tumor necrosis factor-α in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 2008, 51, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Kapadia, S.; Lee, J.; Durand, J.B.; Bies, R.D.; Young, J.B.; Mann, D.L. Tumor necrosis factor-α and tumor necrosis factor receptors in the failing human heart. Circulation 1996, 93, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.L.; Lin, L.; Wang, Y.; Knowlton, A.A. Effect of ovariectomy on cardiac gene expression: Inflammation and changes in SOCS gene expression. Physiol. Genom. 2008, 32, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wietlisbach, V.; Marques-Vidal, P.; Kuulasmaa, K.; Karvanen, J.; Paccaud, F.; Project, W.M. The relation of body mass index and abdominal adiposity with dyslipidemia in 27 general populations of the WHO MONICA Project. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Tzang, B.S.; Kuo, W.W.; Lin, Y.M.; Yang, A.L.; Chen, S.H.; Tsai, F.J.; Wu, F.L.; Lu, M.C.; Huang, C.Y. Cardiac fas receptor-dependent apoptotic pathway in obese Zucker rats. Obesity 2007, 15, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Tzang, B.S.; Kuo, W.W.; Wu, F.L.; Chen, Y.S.; Tsai, C.H.; Huang, C.Y.; Lee, S.D. More activated cardiac mitochondrial-dependent apoptotic pathway in obese Zucker rats. Obesity 2007, 15, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lee, S.D. Possible pathophysiology of heart failure in obesity: Cardiac apoptosis. BioMedicine 2012, 2, 36–40. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Hsieh, P.S.; Cheng, Y.J.; Cheng, S.M.; Chen, C.J.; Huang, C.Y.; Kuo, C.H.; Kao, C.L.; Shyu, W.C.; Lee, S.D. Anti-apoptotic and pro-survival effects of food restriction on high-fat diet-induced obese hearts. Cardiovasc. Toxicol. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Strissel, K.J.; Obin, M.S.; Greenberg, A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009, 150, 2161–2168. [Google Scholar]
- Caliman, I.F.; Lamas, A.Z.; Dalpiaz, P.L.; Medeiros, A.R.; Abreu, G.R.; Figueiredo, S.G.; Gusmao, L.N.; Andrade, T.U.; Bissoli, N.S. Endothelial relaxation mechanisms and oxidative stress are restored by atorvastatin therapy in ovariectomized rats. PLoS ONE 2013, 8, e80892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Lin, Y.Y.; Su, C.T.; Hu, C.C.; Yang, A.L. Improvement of acetylcholine-induced vasodilation by acute exercise in ovariectomized hypertensive rats. Chin. J. Physiol. 2016, 59, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.F.; Brito Jde, O.; Bernardes, N.; Dias Dda, S.; Malfitano, C.; Morris, M.; Llesuy, S.F.; Irigoyen, M.C.; de Angelis, K. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: Autonomic, inflammatory, and oxidative stress evaluations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1532–R1539. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, Y.Y.; Hsu, C.C.; Cheng, S.M.; Shyu, W.C.; Ting, H.; Yang, A.L.; Ho, T.J.; Lee, S.D. Antiapoptotic effect of exercise training on ovariectomized rat hearts. J. Appl. Physiol. 2016, 121, 457–465. [Google Scholar] [CrossRef] [PubMed]






| WKY | SHR-Sham | SHR-OVX | |
|---|---|---|---|
| Number of Animals | 8 | 8 | 8 |
| Body weight (g) | 218 ± 14 | 219 ± 8 | 253 ± 8 **,## |
| Uterine weight (g) | 0.46 ± 0.10 | 0.41 ± 0.09 | 0.08 ± 0.01 **,## |
| Resting HR (bpm) | 374 ± 20 | 458 ± 19 ** | 463 ± 16 ** |
| Heart Weight Index | |||
| WHW (g) | 0.78 ± 0.04 | 0.83 ± 0.04 * | 0.93 ± 0.06 **,## |
| LVW (g) | 0.57 ± 0.05 | 0.61 ± 0.03 | 0.65 ± 0.06 ** |
| LVW (g)/WHW (g) | 0.73 ± 0.03 | 0.73 ± 0.04 | 0.71 ± 0.05 |
| WHW (g)/BW (g) * 103 | 3.57 ± 0.34 | 3.80 ± 0.18 | 3.65 ± 0.18 |
| LVW (g)/BW (g) * 103 | 2.61 ± 0.31 | 2.78 ± 0.18 | 2.58 ± 0.23 |
| WHW (g)/TL (mm) * 103 | 19.87 ± 1.15 | 21.34 ± 0.93 * | 23.64 ± 1.63 **,## |
| LVW (g)/TL (mm) * 103 | 14.53 ± 1.24 | 15.58 ± 0.88 | 16.73 ± 1.85 ** |
| Blood Pressure | |||
| SBP (mmHg) | 121 ± 7 | 169 ± 7 ** | 174 ± 2 ** |
| DBP (mmHg) | 83 ± 12 | 129 ± 10 ** | 134 ± 6 ** |
| MAP (mmHg) | 96 ± 9 | 141 ± 8 ** | 145 ± 6 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-Y.; Cheng, Y.-J.; Hu, J.; Chu, L.-X.; Shyu, W.-C.; Kao, C.-L.; Lin, T.-B.; Kuo, C.-H.; Yang, A.-L.; Lee, S.-D. The Coexistence of Hypertension and Ovariectomy Additively Increases Cardiac Apoptosis. Int. J. Mol. Sci. 2016, 17, 2036. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17122036
Lin Y-Y, Cheng Y-J, Hu J, Chu L-X, Shyu W-C, Kao C-L, Lin T-B, Kuo C-H, Yang A-L, Lee S-D. The Coexistence of Hypertension and Ovariectomy Additively Increases Cardiac Apoptosis. International Journal of Molecular Sciences. 2016; 17(12):2036. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17122036
Chicago/Turabian StyleLin, Yi-Yuan, Yu-Jung Cheng, Jun Hu, Li-Xi Chu, Woei-Cherng Shyu, Chung-Lan Kao, Tzer-Bin Lin, Chia-Hua Kuo, Ai-Lun Yang, and Shin-Da Lee. 2016. "The Coexistence of Hypertension and Ovariectomy Additively Increases Cardiac Apoptosis" International Journal of Molecular Sciences 17, no. 12: 2036. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17122036