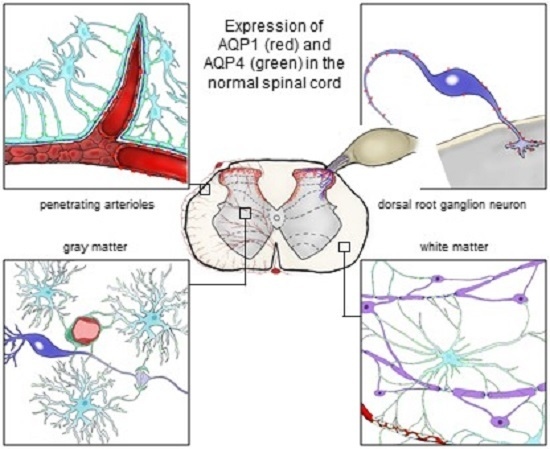
Aquaporins in the Spinal Cord
Abstract
:1. Introduction
2. Expression Pattern of Aquaporins (AQPS) in the Healthy Spinal Cord
2.1. Aquaporin 1 (AQP1)
2.2. Aquaporin 4 (AQP4)
2.3. Aquaporin 9 (AQP9)
3. AQP Expression in Disease Conditions of Spinal Cord
3.1. Aquaporin 1 (AQP1)
3.1.1. AQP1 in Spinal Cord Injury (SCI) and Edema
3.1.2. Changes of AQP1 Expression in Spinal Cord and Dorsal Root Ganglion in Response to Peripheral Nerve and Tissue Damage
3.1.3. AQP1 in Multiple Sclerosis (MS) and Neuromyelitis Optica Spectrum Disorders (NMOsd)
3.2. Aquaporin 4 (AQP4)
3.2.1. AQP4 in Spinal Cord Injury (SCI) and Syringomyelia
3.2.2. AQP4 in Neuromyelitis Optica (NMO) and Multiple Sclerosis (MS)
3.2.3. AQP4 in Amyotrophic Lateral Sclerosis (ALS)
4. Implications of Physiological and Pathophysiological AQP Expression in the Spinal Cord
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Otsuka, T.; Hurn, P.D.; Traystman, R.J.; Haug, F.M.; Froehner, S.C.; Adams, M.E.; Neely, J.D.; Agre, P.; Ottersen, O.P.; et al. An α-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. USA 2003, 100, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Frøkiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels—From atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Ren. Physiol. 2000, 278, F13–F28. [Google Scholar]
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; van Hoek, A.N.; Hediger, M.A. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 1998, 273, 24737–24743. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Praetorius, J.; Frokiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Preston, G.M.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654. [Google Scholar] [PubMed]
- Fujiyoshi, Y.; Mitsuoka, K.; de Groot, B.L.; Philippsen, A.; Grubmüller, H.; Agre, P.; Engel, A. Structure and function of water channels. Curr. Opin. Struct. Biol. 2002, 12, 509–515. [Google Scholar] [CrossRef]
- Yool, A.J.; Weinstein, A.M. New roles for old holes: Ion channel function in aquaporin-1. News Physiol. Sci. 2002, 17, 68–72. [Google Scholar] [PubMed]
- Hub, J.S.; Grubmüller, H.; de Groot, B.L. Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations? In Aquaporins; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–76. [Google Scholar]
- Cui, Y.; Bastien, D.A. Water transport in human aquaporin-4: Molecular dynamics (MD) simulations. Biochem. Biophys. Res. Commun. 2011, 412, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Tajkhorshid, E.; Schulten, K. The mechanism of glycerol conduction in aquaglyceroporins. Structure 2001, 9, 1083–1093. [Google Scholar] [CrossRef]
- Oshio, K.; Binder, D.K.; Yang, B.; Schecter, S.; Verkman, A.S.; Manley, G.T. Expression of aquaporin water channels in mouse spinal cord. Neuroscience 2004, 127, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Yasumura, T.; Hudson, C.S.; Agre, P.; Nielsen, S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA 1998, 95, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Oshio, K.; Watanabe, H.; Song, Y.; Verkman, A.S.; Manley, G.T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005, 19, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Minami, S.; Itoh, S.; Shiraishi, S.; Yokoo, H.; Yanagita, T.; Uezono, Y.; Mohri, M.; Wada, A. Aquaporin subtypes in rat cerebral microvessels. Neurosci. Lett. 2001, 297, 163–166. [Google Scholar] [CrossRef]
- Wilson, A.J.; Carati, C.J.; Gannon, B.J.; Haberberger, R.; Chataway, T.K. Aquaporin-1 in blood vessels of rat circumventricular organs. Cell Tissue Res. 2010, 340, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Oklinski, M.K.; Lim, J.S.; Choi, H.J.; Oklinska, P.; Skowronski, M.T.; Kwon, T.H. Immunolocalization of water channel proteins AQP1 and AQP4 in rat spinal cord. J. Histochem. Cytochem. 2014, 62, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Davies, D.C.; Bell, B.A.; Krishna, S. Increased aquaporin 1 water channel expression in human brain tumours. Br. J. Cancer 2002, 87, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Shields, S.D.; Mazario, J.; Skinner, K.; Basbaum, A.I. Anatomical and functional analysis of aquaporin 1, a water channel in primary afferent neurons. Pain 2007, 131, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Oshio, K.; Watanabe, H.; Yan, D.; Verkman, A.S.; Manley, G.T. Impaired pain sensation in mice lacking Aquaporin-1 water channels. Biochem. Biophys. Res. Commun. 2006, 341, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Verkman, A.S. Aquaporin-1 tunes pain perception by interaction with NaV1.8 Na+ channels in dorsal root ganglion neurons. J. Biol. Chem. 2010, 285, 5896–5906. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [PubMed]
- Maugeri, R.; Schiera, G.; di Liegro, C.M.; Fricano, A.; Iacopino, D.G.; di Liegro, I. Aquaporins and brain tumors. Int. J. Mol. Sci. 2016, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A. Water and nonelectrolyte permeability of lipid bilayer membranes. J. Gen. Physiol. 1976, 68, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Binder, D.K.; Manley, G.T.; Krishna, S.; Verkman, A.S. Molecular mechanisms of brain tumor edema. Neuroscience 2004, 129, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Auguste, K.I.; Manley, G.T.; Verkman, A.S. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J. Cereb. Blood Flow Metab. 2006, 26, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005, 280, 13906–13912. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Krishna, S. Water transport becomes uncoupled from K+ siphoning in brain contusion, bacterial meningitis, and brain tumours: Immunohistochemical case review. J. Clin. Pathol. 2003, 56, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007, 22, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Petit, J.M.; Brunet, J.F.; Magistretti, P.J.; Charriaut-Marlangue, C.; Regli, L. Distribution of Aquaporin 9 in the adult rat brain: Preferential expression in catecholaminergic neurons and in glial cells. Neuroscience 2004, 128, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Lindland, H.; Zelenin, S.; Roberg, B.A.; Gundersen, B.B.; Petersen, P.; Rinvik, E.; Torgner, I.A.; Ottersen, O.P. Brain mitochondria contain aquaporin water channels: Evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 2005, 19, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.M.; Skowronski, M.T.; Füchtbauer, E.M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiaer, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Lee, J.; Unabia, G.C.; Johnson, K.; Ye, Z.; Vergara, L.; Hulsebosch, C.E.; Perez-Polo, J.R. Aquaporin 1–A novel player in spinal cord injury. J. Neurochem. 2008, 105, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Arciénega, I.I.; Brunet, J.F.; Bloch, J.; Badaut, J. Cell locations for AQP1, AQP4 and 9 in the non-human primate brain. Neuroscience 2010, 167, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.I.; Tabunoki, H.; Yamamura, T.; Arima, K.; Konno, H. Human astrocytes express aquaporin-1 and aquaporin-4 in vitro and in vivo. Neuropathology 2007, 27, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tan, M.; Gu, M.; Marshall, C.; Ding, J.; Hu, G.; Xiao, M. Cellular localization of aquaporin-1 in the human and mouse trigeminal systems. PLoS ONE 2012, 7, e46379. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Verkman, A.S. Superresolution imaging of aquaporin-4 cluster size in antibody-stained paraffin brain sections. Biophys. J. 2015, 109, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Vitellaro-Zuccarello, L.; Mazzetti, S.; Bosisio, P.; Monti, C.; de Biasi, S. Distribution of aquaporin 4 in rodent spinal cord: Relationship with astrocyte markers and chondroitin sulfate proteoglycans. Glia 2005, 51, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, T.; Verkman, A.S. cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel. J. Biol. Chem. 1995, 270, 22907–22913. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Moeller, H.B.; Zelenina, M.; Snaebjornsson, M.T.; Holen, T.; MacAulay, N. Differential water permeability and regulation of three aquaporin 4 isoforms. Cell. Mol. Life Sci. 2010, 67, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Furman, C.S.; Gorelick-Feldman, D.A.; Davidson, K.G.V.; Yasumura, T.; Neely, J.D.; Agre, P.; Rash, J.E. Aquaporin-4 square array assembly: Opposing actions of M1 and M23 isoforms. Proc. Natl. Acad. Sci. USA 2003, 100, 13609–13614. [Google Scholar] [CrossRef] [PubMed]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frøkiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Regli, L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience 2004, 129, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Hirt, L.; Granziera, C.; Bogousslavsky, J.; Magistretti, P.J.; Regli, L. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Mylonakou, M.N.; Petersen, P.H.; Rinvik, E.; Rojek, A.; Vladimarsdottir, E.; Zelenin, S.; Zeuthen, T.; Nielsen, S.; Ottersen, O.P.; Amiry-Moghaddam, M. Analysis of mice with targeted deletion of AQP9 gene provides conclusive evidence for expression of AQP9 in neurons. J. Neurosci. Res. 2009, 87, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, C.; Asplund, A.; Catrina, A.; Nielsen, S.; Rützler, M. A systematic characterization of aquaporin-9 expression in human normal and pathological tissues. J. Histochem. Cytochem. 2016, 64, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Tanaka, Y.; Matsuzaki, T.; Morishita, Y.; Ishibashi, K. Aquaporin-11 (AQP11) Expression in the Mouse Brain. Int. J. Mol. Sci. 2016, 17, 861. [Google Scholar] [CrossRef] [PubMed]
- Larivière, R.C.; Nguyen, M.D.; Ribeiro-da-Silva, A.; Julien, J.P. Reduced number of unmyelinated sensory axons in peripherin null mice. J. Neurochem. 2002, 81, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in endothelia. Kidney Int. 2006, 69, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Bhat, R.V.; Preston, G.M.; Guggino, W.B.; Baraban, J.M.; Agre, P. Molecular characterization of an aquaporin cDNA from brain: Candidate osmoreceptor and regulator of water balance. Proc. Natl. Acad. Sci. USA 1994, 91, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Puschmann, T.B.; Dixon, K.J.; Turnley, A.M. Species differences in reactivity of mouse and rat astrocytes in vitro. Neurosignals 2010, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Ahlemeyer, B.; Kehr, K.; Richter, E.; Hirz, M.; Baumgart-Vogt, E.; Herden, C. Phenotype, differentiation, and function differ in rat and mouse neocortical astrocytes cultured under the same conditions. J. Neurosci. Methods 2013, 212, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Srinivas, M.; Li, W.; Brosnan, C.F.; Frigeri, A.; Spray, D.C. New possible roles for aquaporin-4 in astrocytes: Cell cytoskeleton and functional relationship with connexin43. FASEB J. 2005, 19, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.C.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.I.; Ryu, H.J.; Kim, J.E.; Chun, W.; Seo, C.H.; Lee, B.C.; Choi, I.G.; Sheen, S.H.; Kang, T.C. The effects of electrical shock on the expressions of aquaporin subunits in the rat spinal cords. Anat. Cell Biol. 2011, 44, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Fan, Z.K.; Cao, Y.; Yu, D.S.; Zhang, Y.Q.; Wang, Y.S. 2-Methoxyestradiol inhibits the up-regulation of AQP4 and AQP1 expression after spinal cord injury. Brain Res. 2011, 1370, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Fang, B.; Tan, W.F.; Wang, Z.L.; Sun, X.J.; Zhang, Z.L.; Ma, H. miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood–spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats. BMC Neurosci. 2016, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Ozsoy, U.; Demir, N.; Hizay, A.; Suzen, L.B.; Angelov, D.N.; Sarikcioglu, L. Temporal and spatial distribution of the aquaporin 1 in spinal cord and dorsal root ganglia after traumatic injuries of the sciatic nerve. Child’s Nerv. Syst. 2014, 30, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Buffoli, B.; Borsani, E.; Rezzani, R.; Rodella, L.F. Chronic constriction injury induces aquaporin-2 expression in the dorsal root ganglia of rats. J. Anat. 2009, 215, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Lennon, P.V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B.G. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Hinson, S.R.; Pittock, S.J.; Lucchinetti, C.F. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007, 69, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zheng, Y.; Shan, F.; Chen, M.; Fan, Y.; Zhang, B.; Gao, C.; Gao, Q.; Yang, N. Development of a cell-based assay for the detection of anti-aquaporin 1 antibodies in neuromyelitis optica spectrum disorders. J. Neuroimmunol. 2014, 273, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, J.S.; Stergiou, C.; Kilidireas, K.; Zisimopoulou, P.; Thomaidis, T.; Tzartos, S.J. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS ONE 2013, 8, e74773. [Google Scholar] [CrossRef] [PubMed]
- Tüzün, E.; Tzartos, J.; Ekizoğlu, E.; Stergiou, C.; Zisimopoulou, P.; Çoban, A.; Shugaiv, E.; Türkoğlu, R.; Kürtüncü, M.; Baykan, B.; Tzartos, S. Aquaporin-1 antibody in neuromyelitis optica patients. Eur. Neurol. 2014, 72, 271–272. [Google Scholar]
- Metz, I.; Beißbarth, T.; Ellenberger, D.; Pache, F.; Stork, L.; Ringelstein, M.; Aktas, O.; Jarius, S.; Wildemann, B.; Dihazi, H.; et al. Serum peptide reactivities may distinguish neuromyelitis optica subgroups and multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e204. [Google Scholar] [CrossRef] [PubMed]
- Misu, T.; Höftberger, R.; Fujihara, K.; Wimmer, I.; Takai, Y.; Nishiyama, S.; Nakashima, I.; Konno, H.; Bradl, M.; Garzuly, F.; et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013, 125, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Guest, J.D.; Zivadinovic, D.; Narayana, P.A.; Herrera, J.J.; Grill, R.J.; Mokkapati, V.U.L.; Gelman, B.B.; Lee, J. Aquaporins in spinal cord injury: The janus face of aquaporin 4. Neuroscience 2010, 168, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Lee, J.; Ye, Z.; Unabia, G.C.; Rafati, D.; Hulsebosch, C.E.; Perez-Polo, J.R. Acute and chronic changes in aquaporin 4 expression after spinal cord injury. Neuroscience 2006, 143, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Nesic, O.; Lee, J.; Johnson, K.M.; Ye, Z.; Xu, G.Y.; Unabia, G.C.; Wood, T.G.; McAdoo, D.J.; Westlund, K.N.; Hulsebosch, C.E.; et al. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 2005, 95, 998–1014. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.V.; Thornton, E.; Vink, R. Substance P as a mediator of neurogenic inflammation after balloon compression induced spinal cord injury. J. Neurotrauma 2013, 30, 1812–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, A.M.; Li, J.J.; Sun, W.; Yang, D.G.; Yang, M.L.; Du, L.J.; Gu, R.; Gao, F.; Li, J.; Chu, H.Y.; et al. Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord injury. Spinal Cord 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zu, J.; Wang, Y.; Xu, G.; Zhuang, J.; Gong, H.; Yan, J. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014, 116, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Guptarak, J.; Wiktorowicz, J.E.; Sadygov, R.G.; Zivadinovic, D.; Paulucci-Holthauzen, A.A.; Vergara, L.; Nesic, O. The cancer drug tamoxifen: A potential therapeutic treatment for spinal cord injury. J. Neurotrauma 2014, 31, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Vitellaro-Zuccarello, L.; Mazzetti, S.; Madaschi, L.; Bosisio, P.; Fontana, E.; Gorio, A.; de Biasi, S. Chronic erythropoietin-mediated effects on the expression of astrocyte markers in a rat model of contusive spinal cord injury. Neuroscience 2008, 151, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jing, Y.; Yuan, X.; Zhang, X.; Li, B.; Liu, M.; Wang, B.; Li, H.; Liu, S.; Xiu, R. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J. Mol. Neurosci. 2014, 54, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.L.; Wu, Q.B.; Yuan, X.C.; Li, B.W.; Liu, M.M.; Zhang, X.Y.; Liu, S.Y.; Li, H.W.; Xiu, R.J. Microvascular protective role of pericytes in melatonin-treated spinal cord injury in the C57BL/6 mice. Chin. Med. J. 2014, 127, 2808–2813. [Google Scholar] [PubMed]
- Liu, D.; Xu, G.Y.; Pan, E.; McAdoo, D.J. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience 1999, 93, 1383–1389. [Google Scholar] [CrossRef]
- Fan, Z.K.; Wang, Y.F.; Cao, Y.; Zhang, M.C.; Zhang, Z.; Lv, G.; Lu, W.; Zhang, Y.Q. The effect of aminoguanidine on compression spinal cord injury in rats. Brain Res. 2010, 1342, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Zhu, Y.; Diao, Y.; Tao, L.; Yuan, W.; Xiong, X.C. Anti-edema effect of epigallocatechin gallate on spinal cord injury in rats. Brain Res. 2013, 1527, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.D.; Pan, H.; Qiao, L. Sulphoraphane enhances aquaporin-4 expression and decreases spinal cord oedema following spinal cord injury. Brain Inj. 2011, 25, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Yonan, J.M.; Binder, D.K. Aquaporin-4 and spinal cord injury. World J. Neurol. 2016, 6, 1. [Google Scholar] [CrossRef]
- Bilston, L.E.; Stoodley, M.A.; Fletcher, D.F. The influence of the relative timing of arterial and subarachnoid space pulse waves on spinal perivascular cerebrospinal fluid flow as a possible factor in syrinx development. J. Neurosurg. 2010, 112, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J.; Völkel, K.; Bartels, C.J.; Samii, M. Disturbances of cerebrospinal fluid flow attributable to arachnoid scarring cause interstitial edema of the cat spinal cord. Neurosurgery 2001, 48, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Hemley, S.J.; Bilston, L.E.; Cheng, S.; Chan, J.N.; Stoodley, M.A. Aquaporin-4 expression in post-traumatic syringomyelia. J. Neurotrauma 2013, 30, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Oklinski, M.K.; Choi, H.J.; Kwon, T.H. Peripheral nerve injury induces aquaporin-4 expression and astrocytic enlargement in spinal cord. Neuroscience 2015, 311, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Misu, T.; Fujihara, K.; Nakamura, M.; Murakami, K.; Endo, M.; Konno, H.; Itoyama, Y. Loss of aquaporin-4 in active perivascular lesions in neuromyelitis optica: A case report. Tohoku J. Exp. Med. 2006, 209, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Roemer, S.F.; Parisi, J.E.; Lennon, V.A.; Benarroch, E.E.; Lassmann, H.; Bruck, W.; Mandler, R.N.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007, 130, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, M.S.; Lee, S.H.; Kim, S.H.; Jung, I.J.; Takahashi, T.; Misu, T.; Fujihara, K.; Kim, H.J. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Mult. Scler. 2010, 16, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, D.; Ho, V.; Dodd, M.; Dawson, H.N.; Zumwalt, A.C.; Colton, C.A. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann. Neurol. 2008, 148, 825–832. [Google Scholar]
- Takahashi, T.; Fujihara, K.; Nakashima, I.; Misu, T.; Miyazawa, I.; Nakamura, M.; Watanabe, S.; Shiga, Y.; Kanaoka, C.; Fujimori, J.; et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: A study on antibody titre. Brain 2007, 130, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.; Jarius, S.; Littleton, E.; Leite, M.I.; Jacob, S.; Gray, B.; Geraldes, R.; Vale, T.; Jacob, A.; Palace, J.; et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch. Neurol. 2008, 65, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Zeka, B.; Hastermann, M.; Hochmeister, S.; Kögl, N.; Kaufmann, N.; Schanda, K.; Mader, S.; Misu, T.; Rommer, P.; Fujihara, K.; et al. Highly encephalitogenic aquaporin 4-specific T cells and NMO-IgG jointly orchestrate lesion location and tissue damage in the CNS. Acta Neuropathol. 2015, 130, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.V.; Huang, H.; Calabresi, P.A.; Levy, M. Pathogenic aquaporin-4 reactive T cells are sufficient to induce mouse model of neuromyelitis optica. Acta Neuropathol. Commun. 2015, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Fischer, M.T.; Bauer, J.; Felts, P.A.; Smith, K.J.; Misu, T.; Fujihara, K.; Bradl, M.; Lassmann, H. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010, 120, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Suzuki, S.O.; Suenaga, T.; Iwaki, T.; Kira, J.I. Reappraisal of aquaporin-4 astrocytopathy in asian neuromyelitis optica and multiple sclerosis patients. Brain Pathol. 2011, 21, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.; Kirk, J.; Herron, B.; Fitzgerald, U.; McQuaid, S. Absence of aquaporin-4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol. 2007, 113, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Jukkola, P.; Guerrero, T.; Gray, V.; Gu, C. Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity. Acta Neuropathol. Commun. 2013, 1, 70. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, C.; Soyfoo, M.S.; Authelet, M.; de Decker, R.; Bataveljic, D.; Delporte, C.; Pochet, R. Aquaporin-4 overexpression in rat ALS model. Anat. Rec. 2009, 292, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Masaki, K.; Yamasaki, R.; Imamura, S.; Suzuki, S.O.; Hayashi, S.; Sato, S.; Nagara, Y.; Kawamura, M.F.; Kira, J. Extensive dysregulations of oligodendrocytic and astrocytic connexins are associated with disease progression in an amyotrophic lateral sclerosis mouse model. J. Neuroinflamm. 2014, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Maletzki, I.; Hülsmann, S.; Holtmann, B.; Schulz-Schaeffer, W.; Kirchhoff, F.; Bähr, M.; Neusch, C. Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2006, 99, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Bataveljić, D.; Nikolić, L.; Milosević, M.; Todorović, N.; Andjus, P.R. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1G93A rat model. Glia 2012, 60, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.S.; Robbins, R.J.; Naftolin, F. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992, 327, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Trotti, D.; Aoki, M.; Pasinelli, P.; Berger, U.V.; Danbolt, N.C.; Brown, R.H.; Hediger, M.A. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J. Biol. Chem. 2001, 276, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, C.; Soyfoo, M.S.; Delporte, C.; Pochet, R. Aquaporin-4 as a potential marker of BBB disruption in ALS models. Amyotroph. Lateral Scler. 2010, 11, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Saporta, S.; Sanberg, P.R. Implications of blood-brain barrier disruption in ALS. Amyotroph. Lateral Scler. 2008, 9, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Sanberg, P.R. Blood-CNS barrier impairment in ALS patients versus an animal model. Front. Cell. Neurosci. 2014, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Saporta, S.; Haller, E.; Kolomey, I.; Bennett, S.P.; Potter, H.; Sanberg, P.R. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE 2007, 2, e1205. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Deane, R.; Ali, Z.; Parisi, M.; Shapovalov, Y.; Banion, O.; Stojanovic, K.; Sagare, A.; Boillee, S.; Cleveland, D.W.; et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008, 11, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Ohta, Y.; Nagai, M.; Morimoto, N.; Kurata, T.; Takehisa, Y.; Ikeda, Y.; Matsuura, T.; Abe, K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J. Neurosci. Res. 2011, 89, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Nichols, C.G.; Maldonado, H.M.; Baksi, K. Downregulation of Kir4. 1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 2007, 281, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Taniguchi, K.; Kofuji, P. Heterogeneity of Kir4. 1 channel expression in glia revealed by mouse transgenesis. Gila 2009, 1715, 1706–1715. [Google Scholar]
- Van Damme, P.; Bogaert, E.; Dewil, M.; Hersmus, N.; Kiraly, D.; Scheveneels, W.; Bockx, I.; Braeken, D.; Verpoorten, N.; Verhoeven, K.; et al. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc. Natl. Acad. Sci. USA 2007, 104, 14825–14830. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, Y.X.; Dou, F.F.; Lü, H.Z.; Ma, Z.W.; Lu, P.H.; Xu, X.M. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem. Int. 2010, 56, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Benesova, J.; Rusnakova, V.; Honsa, P.; Pivonkova, H.; Dzamba, D.; Kubista, M.; Anderova, M. Distinct expression/function of potassium and chloride channels contributes to the diverse volume regulation in cortical astrocytes of GFAP/EGFP mice. PLoS ONE 2012, 7, e29725. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.N.; Sun, X.L.; Gao, L.; Fan, Y.; Ding, J.H.; Hu, G. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol. Cell. Neurosci. 2007, 34, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gunnarson, E.; Zelenina, M.; Axehult, G.; Song, Y.; Bondar, A.; Krieger, P.; Brismar, H.; Zelenin, S.; Aperia, A. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia 2008, 596, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Inquimbert, P.; Bartels, K.; Babaniyi, O.B.; Barrett, L.B.; Tegeder, I.; Scholz, J. Peripheral nerve injury produces a sustained shift in the balance between glutamate release and uptake in the dorsal horn of the spinal cord. Pain 2012, 29, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Hughes, M.G.; Ye, Z.; Hulsebosch, C.E.; Mcadoo, D.J. Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Exp. Neurol. 2004, 187, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lunde, L.K.; Nuntagij, P.; Oguchi, T.; Camassa, L.M.A.; Nilsson, L.N.G.; Lannfelt, L.; Xu, Y.; Amiry-Moghaddam, M.; Ottersen, O.P.; et al. Loss of astrocyte polarization in the Tg-ArcSwe mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 711–722. [Google Scholar]
- Wolburg-buchholz, K.; Mack, A.F.; Engelhardt, B.; Wolburg, H.; Pfeiffer, F.; Steiner, E. Loss of astrocyte polarity marks blood—Brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol 2009, 118, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Misu, T.; Fujihara, K.; Kakita, A.; Konno, H.; Nakamura, M.; Watanabe, S.; Nakashima, I. Loss of aquaporin 4 in lesions of neuromyelitis optica: Distinction from multiple sclerosis. Brain 2007, 130, 1224–1234. [Google Scholar] [CrossRef] [PubMed]


| Spinal Cord | Brain | |||
|---|---|---|---|---|
| Aquaporin (AQP) | Cell Type and/or Structure | Detection Method and Reference | Cell Type and/or Structure | Detection Method and Reference |
| AQP1 | Unmyelinated sensory fibers in DH Myelinated neuronal fibers (sparse) in DH (#) Lamina V and X Endothelial cells of small penetrating arterioles in immediate vicinity to the glia limitans (sparse, #) Astrocytes and ependymal cells (#) | IHC, RT-PCR, WB [23,24,39] IHC [23] IHC [14,21] IHC [21] IHC [39] | Epithelial cells of choroid plexus Endothelial cells of circumventricular organs Endothelial cells in blood vessels in brain parenchyma (sparse, #) Astrocytes, schwann cells surrounding the oculomotor and trigeminal cranial nerve fibers, neurons on the surface of the pia blood vessels (#) | WB, IHC, IMEM [16,18] IHC, RT-PCR [20] IHC [20,22] IHC [40,41,42] |
| AQP4 | Astrocytes end-feet encircling capillaries, and building up the glia limitans, processes enveloping myelinated neuronal fibers; Expression polarized to foot-processes in protoplasmatic astrocytes and more evenly distributed in fibrous astrocytes | IHC, RT-PCR, WB [14,21,43,44] | Subpial astrocytes processes forming glia limitans, perivascular astrocyte endfeet in cortex; expression highly polarized to astrocytes foot-processes Basolateral membrane of ependymal cells | IHC, IMEM, NB, RT-PCR, WB, [17,26,43,45,46,47,48] WB, IHC, IMEM [17,26] |
| AQP9 | Astrocyte processes in white matter and glia limitans (#) | IHC, RT-PCR, WB [14,40] | Neurons, ependymal cells (#), tanycytes (#) Astrocytes mitochondria and astrocytes in the immediate vicinity to subarachnoid space and ventricles (#) | IHC, RT-PCR, WB [37,38,40,49,50,51,52,53] IHC, RT-PCR [36,37,38,52] |
| AQP11 | Not investigated | Not investigated | Dendrites of Purkinje cells in cerebellum, neurons in hippocampus and cerebral cortex (#) Epithelium of the choroid plexus and at the endothelium of the brain capillary (#) | IHC, NB, RT-PCR, WB [15,54] IHC, RT-PCR, WB [55] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oklinski, M.K.; Skowronski, M.T.; Skowronska, A.; Rützler, M.; Nørgaard, K.; Nieland, J.D.; Kwon, T.-H.; Nielsen, S. Aquaporins in the Spinal Cord. Int. J. Mol. Sci. 2016, 17, 2050. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17122050
Oklinski MK, Skowronski MT, Skowronska A, Rützler M, Nørgaard K, Nieland JD, Kwon T-H, Nielsen S. Aquaporins in the Spinal Cord. International Journal of Molecular Sciences. 2016; 17(12):2050. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17122050
Chicago/Turabian StyleOklinski, Michal K., Mariusz T. Skowronski, Agnieszka Skowronska, Michael Rützler, Kirsten Nørgaard, John D. Nieland, Tae-Hwan Kwon, and Søren Nielsen. 2016. "Aquaporins in the Spinal Cord" International Journal of Molecular Sciences 17, no. 12: 2050. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17122050