Endogenously Expressed IL-4Rα Promotes the Malignant Phenotype of Human Pancreatic Cancer In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Expression of Type-II IL-4R Chains in Pancreatic Cancer Cells
2.2. Effect of IL-4Rα Inhibition on Basal In Vitro Cell Growth
2.3. Effect of IL-4Rα Inhibition on Cancer Cell Motility
2.4. Effect of IL-4Rα Downregulation on IL-4 and IL-13 Signaling
2.5. Inhibition of STAT3 Results in Reduced Tumor Cell Survival
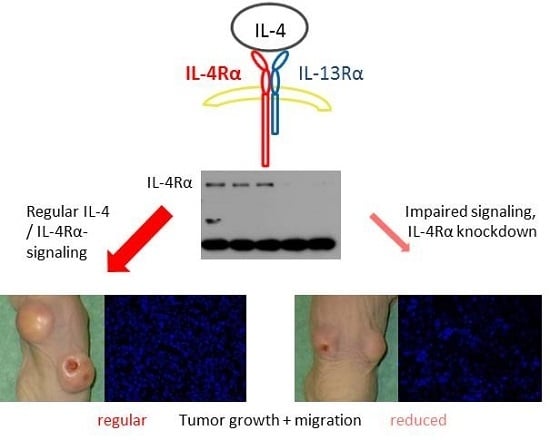
2.6. Suppression of IL-4Rα Inhibits Tumor Growth In Vivo
2.7. Morphology and Immunohistochemical Staining of Xenograft Tumors
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection
4.3. Immunoblotting
4.4. Cell Growth Assays
4.5. Cell Cycle Analysis
4.6. Cell Migration Assay
4.7. Single-Cell Movement Assay
4.8. In Vivo Tumorigenicity Assay
4.9. Microscopic Analysis of Xenograft Tumors
4.10. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL-4/-13 | Interleukin-4/-13 |
| IL-4Rα | Interleukin-4-receptor-α-chain |
| IL-13Rα1 | Interleukin-13-receptor-α-1-chain |
| LLL12 | Specific inhibitor of STAT3 phosphorylation |
| N9, N10 | Capan-1 cells carrying a negative control plasmid |
| 2-11, 3-20 | Capan-1 cells transfected with shRNA targeting IL-4Rα |
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Kawakami, M.; Husain, S.R.; Puri, R.K. Targeting Interleukin-4 receptors for effective pancreatic cancer therapy. Cancer Res. 2002, 62, 3575–3580. [Google Scholar] [PubMed]
- Kornmann, M.; Kleeff, J.; Debinski, W.; Korc, M. Pancreatic cancer cells express Interleukin-13 and -4 receptors, and their growth is inhibited by pseudomonas exotoxin coupled to Interleukin-13 and -4. Anticancer Res. 1999, 19, 125–131. [Google Scholar] [PubMed]
- Paul, W.E. Interleukin-4: A prototypic immunoregulatory lymphokine. Blood 1991, 77, 1859–1870. [Google Scholar] [PubMed]
- Murata, T.; Obiri, N.I.; Puri, R.K. Structure of and signal transduction through Interleukin-4 and Interleukin-13 Receptors (Review). Int. J. Mol. Med. 1998, 1, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.A.; Grandis, J.R. STAT3 signaling: Anticancer strategies and challenges. Mol. Interv. 2011, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Watanabe, M.; Hayashi, A.; Nakazawa, A.; Yajima, T.; Okazawa, A.; Yamazaki, M.; Ishii, H.; Hibi, T. Regulatory effect of Interleukin-4 and Interleukin-13 on colon cancer cell adhesion. Br. J. Cancer 2000, 82, 1717–1723. [Google Scholar] [PubMed]
- Todaro, M.; Lombardo, Y.; Francipane, M.G.; Alea, M.P.; Cammareri, P.; Iovino, F.; Di Stefano, A.B.; di Bernardo, C.; Agrusa, A.; Condorelli, G.; et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived Interleukin-4. Cell Death Differ. 2008, 15, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Prokopchuk, O.; Liu, Y.; Henne-Bruns, D.; Kornmann, M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: Evidence for autocrine and paracrine actions. Br. J. Cancer 2005, 92, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, J.; Wang, Z.; Zhang, J.; Xiao, M.; Wang, C.; Lu, Y.; Qin, Z. Endogenous Interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008, 68, 8687–8694. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, M.; Modlin, R.L.; Ohmen, J.D.; Moy, R.L. Local expression of antiinflammatory cytokines in cancer. J. Clin. Investig. 1993, 91, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Obiri, N.I.; Hillman, G.G.; Haas, G.P.; Sud, S.; Puri, R.K. Expression of high affinity Interleukin-4 Receptors on human renal cell carcinoma cells and inhibition of tumor cell growth in vitro by Interleukin-4. J. Clin. Investig. 1993, 91, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Yuzuki, D.H.; Lin, R.T.; Foshag, L.J.; Morton, D.L.; Hoon, D.S. Interleukin 4 receptor expression and growth inhibition of gastric carcinoma cells by Interleukin 4. Cancer Res. 1992, 52, 6059–6065. [Google Scholar] [PubMed]
- Fallon, P.G.; Jolin, H.E.; Smith, P.; Emson, C.L.; Townsend, M.J.; Fallon, R.; Smith, P.; McKenzie, A.N. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 2002, 17, 7–17. [Google Scholar] [CrossRef]
- Goldstein, R.; Hanley, C.; Morris, J.; Cahill, D.; Chandra, A.; Harper, P.; Chowdhury, S.; Maher, J.; Burbridge, S. Clinical investigation of the role of Interleukin-4 and Interleukin-13 in the evolution of prostate cancer. Cancers 2011, 3, 4281–4293. [Google Scholar] [CrossRef]
- Joshi, B.H.; Leland, P.; Lababidi, S.; Varrichio, F.; Puri, R.K. Interleukin-4 receptor α overexpression in human bladder cancer correlates with the pathological grade and stage of the disease. Cancer. Med. 2014, 3, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Bader, A.; Winter, D.; Rodig, S.J.; Bueno, R.; Sugarbaker, D.J. Expression of Interleukin-4 receptor α in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin. Cancer Res. 2012, 18, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Venmar, K.; Carter, K.; Hwang, D.; Dozier, E.; Fingleton, B. IL4 receptor ILR4a regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res. 2014, 74, 4329–4340. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Qin, Z. Paradoxical roles of IL-4 in tumor immunity. Cell. Mol. Immunol. 2009, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.L.; Gonzalez-Hernandez, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and genotype of pancreatic cancer cell lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Roca, H.; Craig, M.J.; Ying, C.; Varsos, Z.S.; Czarnieski, P.; Alva, A.S.; Hernandez, J.; Fuller, D.; Daignault, S.; Healy, P.N.; et al. IL-4 Induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin up-regulation. J. Cell. Biochem. 2012, 113, 1569–1580. [Google Scholar] [PubMed]
- Todaro, M.; Alea, M.P.; di Stefano, A.B.; Cammareri, P.; Vermeulen, L.; Iovino, F.; Tripodo, C.; Russo, A.; Gulotta, G.; Medema, J.P.; et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of Interleukin-4. Cell Stem Cell 2007, 1, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Bhardwaj, A.; Singh, S.; Srivastava, S.; McClellan, S.; Nirodi, C.; Piazza, G.; Grizzle, W.; Owen, L.; Singh, A. An undesired effect of chemotherapy: Gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor κB- and hypoxia-inducible factor 1α-mediated up-regulation of CXCR4. J. Biol. Chem. 2013, 288, 21197–21207. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Kunz, C.; Rudloff, S. Inhibition of pancreatic cancer cell migration by plasma anthocyanins isolated from healthy volunteers receiving an anthocyanin-rich berry juice. Eur. J. Nutr. 2017, 56, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Formentini, A.; Prokopchuk, O.; Strater, J.; Kleeff, J.; Grochola, L.F.; Leder, G.; Henne-Bruns, D.; Korc, M.; Kornmann, M. Interleukin-13 exerts autocrine growth-promoting effects on human pancreatic cancer, and its expression correlates with a propensity for lymph node metastases. Int. J. Colorectal Dis. 2009, 24, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O.; et al. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.; Heinze, S.; Detjen, K.M.; Peters, M.; Welzel, M.; Hauff, P.; Schirner, M.; Wiedenmann, B.; Rosewicz, S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology 2003, 125, 891–905. [Google Scholar] [CrossRef]
- Wang, S.W.; Sun, Y.M. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review). Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [PubMed]
- He, S.; Liao, G.; Liu, Y.; Huang, L.; Kang, M.; Chen, L. Overexpression of STAT3/pSTAT3 was associated with poor prognosis in gastric cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 20014–20023. [Google Scholar] [PubMed]
- Carpenter, R.; Lo, H. STAT3 target genes relevant to human cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hutzen, B.; Li, P.K.; Ball, S.; Zuo, M.; DeAngelis, S.; Foust, E.; Sobo, M.; Friedman, L.; Bhasin, D.; et al. A novel small molecule, LLL12, inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells. Neoplasia 2010, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, W.; Collins, M.A.; Bednar, F.; Rakshit, S.; Zetter, B.R.; Stanger, B.Z.; Chung, I.; Rhim, A.D.; di Magliano, M.P. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013, 73, 6359–6374. [Google Scholar] [CrossRef] [PubMed]
- Erkan, M.; Adler, G.; Apte, M.V.; Bachem, M.G.; Buchholz, M.; Detlefsen, S.; Esposito, I.; Friess, H.; Gress, T.M.; Habisch, H.J.; et al. StellaTUM: Current consensus and discussion on pancreatic stellate cell research. Gut 2012, 61, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Poulikakos, P.I.; Solit, D.B. Resistance to MEK inhibitors: Should we co-target upstream? Sci. Signal. 2011, 4, pe16. [Google Scholar] [CrossRef] [PubMed]
- Palagani, V.; Bozko, P.; El Khatib, M.; Belahmer, H.; Giese, N.; Sipos, B.; Malek, N.P.; Plentz, R.R. Combined inhibition of Notch and JAK/STAT is superior to monotherapies and impairs pancreatic cancer progression. Carcinogenesis 2014, 35, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, M.; Arber, N.; Korc, M. Inhibition of basal and mitogen-stimulated pancreatic cancer cell growth by cyclin D1 Antisense is associated with loss of tumorigenicity and potentiation of cytotoxicity to cisplatinum. J. Clin. Investig. 1998, 101, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhou, S.; Siech, M.; Habisch, H.; Seufferlein, T.; Bachem, M.G. Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway. Br. J. Cancer 2014, 110, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Brockschmidt, C.; Hirner, H.; Huber, N.; Eismann, T.; Hillenbrand, A.; Giamas, G.; Radunsky, B.; Ammerpohl, O.; Bohm, B.; Henne-Bruns, D.; et al. Anti-apoptotic and growth-stimulatory functions of CK1 Delta and epsilon in ductal adenocarcinoma of the pancreas are inhibited by IC261 in vitro and in vivo. Gut 2008, 57, 799–806. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traub, B.; Sun, L.; Ma, Y.; Xu, P.; Lemke, J.; Paschke, S.; Henne-Bruns, D.; Knippschild, U.; Kornmann, M. Endogenously Expressed IL-4Rα Promotes the Malignant Phenotype of Human Pancreatic Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040716
Traub B, Sun L, Ma Y, Xu P, Lemke J, Paschke S, Henne-Bruns D, Knippschild U, Kornmann M. Endogenously Expressed IL-4Rα Promotes the Malignant Phenotype of Human Pancreatic Cancer In Vitro and In Vivo. International Journal of Molecular Sciences. 2017; 18(4):716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040716
Chicago/Turabian StyleTraub, Benno, Lie Sun, Yongsu Ma, Pengfei Xu, Johannes Lemke, Stephan Paschke, Doris Henne-Bruns, Uwe Knippschild, and Marko Kornmann. 2017. "Endogenously Expressed IL-4Rα Promotes the Malignant Phenotype of Human Pancreatic Cancer In Vitro and In Vivo" International Journal of Molecular Sciences 18, no. 4: 716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040716