High Glucose Accelerates Cell Proliferation and Increases the Secretion and mRNA Expression of Osteopontin in Human Pancreatic Duct Epithelial Cells
Abstract
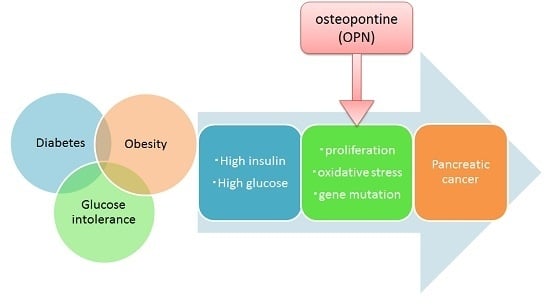
:1. Introduction
2. Results
2.1. Cell Proliferation
2.2. Quantification of OPN mRNA
2.3. OPN Protein Expression Analysis
2.4. OPN Gene Inhibition Using OPN-siRNA
2.5. 8-OHdG Detection
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Evaluation of Cell Proliferation
4.3. RNA/DNA/Protein Extraction
4.4. Real-Time PCR
4.5. Protein Detection
4.6. Gene Inhibition Experiment Using OPN-Small Interfering RNA (siRNA)
4.7. Detection of 8-Hydroxydeoxyguanosine (8-OHdG) in Different Concentrations of Glucose
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Japan Pancreas Society. Pancreatic Cancer Registry Report 2007. Suizo 2007, 22, e54. [Google Scholar]
- Magruder, J.T.; Elahi, D.; Andersen, D.K. Diabetes and pancreatic cancer: Chicken or egg? Pancreas 2011, 40, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M. Pancreatic cancer-associated diabetes mellitus: Prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008, 134, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Ansary-Moghaddam, A.; Berrington de González, A.; Berrington de González, A.; Barzi, F.; Woodward, M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br. J. Cancer 2005, 92, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.; Wright, D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995, 273, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Gapstur, S.M.; Gann, P.H.; Lowe, W.; Liu, K.; Colangelo, L.; Dyer, A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000, 283, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Yokote, K.; Yamazaki, M.; Ridall, A.L.; Butler, W.T.; Matsumoto, T.; Tamura, K.; Saito, Y.; Mori, S. Enhanced expression of osteopontin by high glucose in cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 1999, 258, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.S.; Zhong, W.B.; Yu, S.C.; Lin, J.Y.; Chi, W.M.; Lee, H.M. Dipyridamole suppresses high glucose-induced osteopontin secretion and mRNA expression in rat aortic smooth muscle cells. Circ. J. 2010, 74, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Catalán, V.; Ramírez, B.; Rodríguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W.; Zeyda, M.; Todoric, J.; Huber, J.; Geyeregger, R.; Weichhart, T.; Aszmann, O.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; et al. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 2008, 149, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, T.; Perez-Tilve, D.; Ogawa, D.; Gizard, F.; Zhao, Y.; Heywood, E.B.; Jones, K.L.; Kawamori, R.; Cassis, L.A.; Tschöp, M.H.; et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Investig. 2007, 117, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W.; Neschen, S.; Pfau, B.; Legerer, B.; Neuhofer, A.; Kahle, M.; Hrabé de Angelis, M.; Schlederer, M.; Mair, M.; Kenner, L.; et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia 2011, 54, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Mass, A.; Al-Ghawas, D.; Shareif, N.; Zghoul, N.; Melhem, M.; Hasan, A.; Al-Ghimlas, F.; Dermime, S.; Behbehani, K. Interaction of osteopontin with IL-18 in obese individuals: Implications for insulin resistance. PLoS ONE 2013, 8, e63944. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.W.; Ou, Y.H.; Peng, S.Y.; Liu, S.H.; Lai, P.L.; Lee, P.H.; Sheu, J.C.; Chen, C.L.; Hsu, H.C. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer 2003, 98, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Yochim, J.; Brabender, J.; Uchida, K.; Danenberg, K.D.; Metzger, R.; Schneider, P.M.; Salonga, D.; Hölscher, A.H.; Danenberg, P.V. Osteopontin but not osteopontin messenger RNA expression is a prognostic marker in curatively resected non-small cell lung cancer. Clin. Cancer Res. 2004, 10, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Rudland, P.S.; Platt-Higgins, A.; El-Tanani, M.; de Silva Rudland, S.; Barraclough, R.; Winstanley, J.H.; Howitt, R.; West, C.R. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002, 62, 3417–3427. [Google Scholar] [PubMed]
- Poruk, K.E.; Firpo, M.A.; Scaife, C.L.; Adler, D.G.; Emerson, L.L.; Boucher, K.M.; Mulvihill, S.J. Serum osteopontin and tissue inhibitor of metalloproteinase 1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma. Pancreas 2013, 42, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Galasso, R.; Matveyenko, A.; Rizza, R.A.; Dry, S.; Butler, P.C. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia 2010, 53, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Parsa, I.; Longnecker, D.S.; Scarpelli, D.G.; Pour, P.; Reddy, J.K.; Lefkowitz, M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985, 45, 1285–1290. [Google Scholar] [PubMed]
- Wagner, M.; Weber, C.K.; Bressau, F.; Greten, F.R.; Stagge, V.; Ebert, M.; Leach, S.D.; Adler, G.; Schmid, R.M. Transgenic overexpression of amphiregulin induces a mitogenic response selectively in pancreatic duct cells. Gastroenterology 2002, 122, 1898–1912. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yeung, S.C.; Hassan, M.M.; Konopleva, M.; Abbruzzese, J.L. Antidiabetic Therapies Affect Risk of Pancreatic Cancer. Gastroenterology 2009, 137, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Zakikhani, M.; Dowling, R.; Fantus, I.G.; Sonenberg, N.; Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006, 66, 10269–10273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X. Do diabetes drugs modify the risk of pancreatic cancer? Gastroenterology 2009, 137, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, Y.; Dai, Z.; Sun, Y. Intermittent high glucose enhances proliferation of vascular smooth muscle cells by upregulating osteopontin. Mol. Cell. Endocrinol. 2009, 313, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.N.; Arffa, M.L.; Chang, V.; Blackwell, R.H.; Syn, W.K.; Zhang, J.; Mi, Z.; Kuo, P.C. Osteopontin-A Master Regulator of Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, E39. [Google Scholar] [CrossRef] [PubMed]
- Chipitsyna, G.; Gong, Q.; Anandanadesan, R.; Alnajar, A.; Batra, S.K.; Wittel, U.A.; Cullen, D.M.; Akhter, M.P.; Denhardt, D.T.; Yeo, C.J. Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells. Int. J. Cancer 2009, 125, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Giardino, I.; Edelstein, D.; Brownlee, M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J. Clin. Investig. 1996, 97, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [PubMed]
- Cowey, S.; Hardy, R.W. The metabolic syndrome: A high-risk state for cancer? Am. J. Pathol. 2006, 169, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Duguid, W.P.; Rosenberg, L.; Viallet, J.; Galloway, D.A.; Tsao, M.S. Long-Term Culture and Immortalization of Epithelial Cells from Normal Adult Human Pancreatic Ducts Transfected by the E6E7 Gene of Human Papilloma Virus 16. Am. J. Pathol. 1996, 148, 1763–1770. [Google Scholar] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, M.; Makino, N.; Matsuda, A.; Ikeda, Y.; Kakizaki, Y.; Saito, Y.; Ueno, Y.; Kawata, S. High Glucose Accelerates Cell Proliferation and Increases the Secretion and mRNA Expression of Osteopontin in Human Pancreatic Duct Epithelial Cells. Int. J. Mol. Sci. 2017, 18, 807. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040807
Ito M, Makino N, Matsuda A, Ikeda Y, Kakizaki Y, Saito Y, Ueno Y, Kawata S. High Glucose Accelerates Cell Proliferation and Increases the Secretion and mRNA Expression of Osteopontin in Human Pancreatic Duct Epithelial Cells. International Journal of Molecular Sciences. 2017; 18(4):807. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040807
Chicago/Turabian StyleIto, Miho, Naohiko Makino, Akiko Matsuda, Yushi Ikeda, Yasuharu Kakizaki, Yoshihiko Saito, Yoshiyuki Ueno, and Sumio Kawata. 2017. "High Glucose Accelerates Cell Proliferation and Increases the Secretion and mRNA Expression of Osteopontin in Human Pancreatic Duct Epithelial Cells" International Journal of Molecular Sciences 18, no. 4: 807. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18040807