Effect of Light- and Dark-Germination on the Phenolic Biosynthesis, Phytochemical Profiles, and Antioxidant Activities in Sweet Corn (Zea mays L.) Sprouts
Abstract
:1. Introduction
2. Results
2.1. Effect of Germination on Moisture Content of Samples
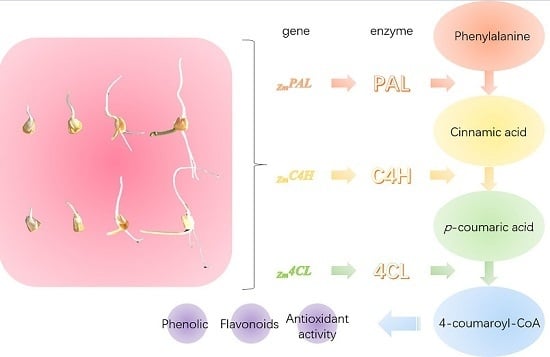
2.2. Effect of Germination on Relative Gene Expression Profiles of Phenolic Biosynthesis
2.3. Effect of Germination on Total Phenolic Content (TPC) in Light and Dark Treatments
2.4. Effect of Germination on TFC in Light and Dark Treatments
2.5. Effect of Germination on Phenolic Profiles in Light and Dark Treatments
2.6. Effect of Germination on Antioxidant Activity in Light and Dark Treatments
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Germination Conditions
4.3. Moisture Content Measurement
4.4. RNA Extractionand Gene Expression Quantitative Analysis
4.5. Phytochemical Extraction
4.6. Determination of Phenolics
4.7. Determination of Flavonoids
4.8. Phenolic Compounds Analysis by HPLC
4.9. Antioxidant Activity Assay
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.; Wang, H.; Zhang, X.; Yang, S.T. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit. Rev. Food Sci. Nutr. 2014, 54, 1180–1201. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [PubMed]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Song, J.F.; Liu, C.Q.; Li, D.J.; Meng, L.L. Effect of cooking methods on total phenolic and carotenoid amounts and DPPH radical scavenging activity of fresh and frozen sweet corn (Zea mays) kernels. Czech J. Food Sci. 2013, 31, 607–612. [Google Scholar]
- Sylvia M., Burwell; Thomas J., Vilsack; U.S. Department of Health and Human Services; U.S. Department of Agriculture. Dietary Guidelines for Americans 2015–2020, Eighth Edition. Available online: www. dietaryguidelines.gov (accessed on 9 June 2017).
- Fahey, J.W.; Zhang, Y.S.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Corke, H. Thermal treatments affect the polyphenol profile and increase antioxidant capacity in five varieties of edible bean milks. Int. J. Food Sci. Technol. 2016, 51, 954–961. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Haileslassie, H.A.; Henry, C.J.; Tyler, R.T. Impact of household food processing strategies on antinutrient (phytate, tannin and polyphenol) contents of chickpeas (Cicer arietinum L.) and beans (Phaseolus vulgaris L.): A review. Int. J. Food Sci. Technol. 2016, 51, 1947–1957. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Tang, X.; Deng, Y.; Liu, L.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-C.; Sun, J.-T. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J. Funct. Foods 2014, 7, 298–304. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Lai, Q.; Li, T.; Fu, X.; Guo, X.; Liu, R.H. The dynamic changes of ascorbic acid, tocopherols and antioxidant activity during germination of soya bean (Glycine max). Int. J. Food Sci. Technol. 2015, 50, 2367–2374. [Google Scholar] [CrossRef]
- Majid, I.; Dhatt, A.S.; Sharma, S.; Nayik, G.A.; Nanda, V. Effect of sprouting on physicochemical, antioxidant and flavonoid profile of onion varieties. Int. J. Food Sci. Technol. 2016, 51, 317–324. [Google Scholar] [CrossRef]
- Duenas, M.; Martinez-Villaluenga, C.; Limon, R.I.; Penas, E.; Frias, J. Effect of germination and elicitation on phenolic composition and bioactivity of kidney beans. Food Res. Int. 2015, 70, 55–63. [Google Scholar] [CrossRef]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; O′Brien, N.M. Levels of potential bioactive compounds including carotenoids, vitamin C and phenolic compounds, and expression of their cognate biosynthetic genes vary significantly in different varieties of potato (Solanum tuberosum L.) grown under uniform cultural conditions. J. Sci. Food Agric. 2016, 96, 1018–1026. [Google Scholar] [PubMed]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Niu, M.; Wang, R.; Chen, Z. Effect of additives on flavonoids, d-chiro-Inositol and trypsin inhibitor during the germination of tartary buckwheat seeds. J. Cereal Sci. 2013, 58, 348–354. [Google Scholar] [CrossRef]
- Yu, D.; Bu, F.; Hou, J.; Kang, Y.; Yu, Z. A morel improved growth and suppressed Fusarium infection in sweet corn. World J. Microbiol. Biotechnol. 2016, 32, 192. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Shetty, K. Developmental stimulation of total phenolics and related antioxidant activity in light- and dark-germinated corn by natural elicitors. Process Biochem. 2005, 40, 1721–1732. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Wang, X.; Gu, Z.; Beta, T. Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Winkelshirley, B. Flavonoid biosynthesis. A Colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.Z.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Sritongtae, B.; Sangsukiam, T.; Morgan, M.R.A.; Duangmal, K. Effect of acid pretreatment and the germination period on the composition and antioxidant activity of rice bean (Vigna umbellata). Food Chem. 2017, 227, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Verma, D.P. Phenolic compounds as regulators of gene expression in plant-microbe relations. Mol. Plant Microbe Interact. 1990, 3, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Hu, T.; Zhang, S.; Zhang, Y.; Zhao, T.; Yu, H.; Kang, Y. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Seo, J.-M.; Arasu, M.V.; Kim, Y.-B.; Park, S.U.; Kim, S.-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015, 177, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Jiang, L.; Liu, Y.; Lv, Y.; Dai, H.; Zhao, H. Genome-wide identification of housekeeping genes in maize. Plant Mol. Biol. 2014, 86, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Messias, R.d.S.; dos Anjos e Silva, S.D.; Rombaldi, C.V. Selection of reliable reference genes for quantitative real-time polymerase chain reaction studies in maize grains. Plant Cell Rep. 2013, 32, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Camino, C.; Conde, R.; Ovsenek, N.; Villanueva, M.A. Actin expression is induced and three isoforms are differentially expressed during germination in Zea mays. J. Exp. Bot. 2005, 56, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Liu, R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 2015, 174, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Sintara, M.; Chang, T. Multi-radical (ORACMR5) antioxidant capacity of selected berries and effects of food processing. J. Berry Res. 2016, 6, 159–173. [Google Scholar] [CrossRef]





| Phenolic Compounds (mg/100 g DW) | Germination Stages | Light | Dark | ||||
|---|---|---|---|---|---|---|---|
| Free Form | Bound Form | Total | Free Form | Bound Form | Total | ||
| Gallic acid | Radicle | 83.33 ± 20.48a | 26.20 ± 0.31a | 109.5 ± 20.8a | 81.84 ± 16.17a | 28.96 ± 1.10a | 110.8 ± 15.1a |
| Germinal | 115.36 ± 1.84a | 33.09 ± 0.80a | 135.4 ± 7.2b | 75.14 ± 11.50a | 36.16 ± 6.16a | 111.3 ± 10.8a | |
| Fibril | 93.10 ± 3.68ab | 65.19 ± 11.89b | 158.3 ± 14.8b | 79.06 ± 15.37a | 64.10 ± 7.05b | 143.2 ± 20.3ab | |
| Euphylla | 95.17 ± 20.93ab | 74.40 ± 3.04b | 169.6 ± 18.1b | 93.83 ± 16.13a | 63.88 ± 6.02b | 157.7 ± 13.7b | |
| Chlorogenic acid | Radicle | 16.92 ± 0.18a | nd | 16.92 ± 0.18a | 16.39 ± 0.40a | nd | 16.39 ± 0.40a |
| Germinal | 25.59 ± 0.78b | nd | 25.59 ± 0.78b | 22.00 ± 0.22b | nd | 22.00 ± 0.22b | |
| Fibril | 29.88 ± 1.74c | nd | 29.88 ± 1.74c | 29.33 ± 0.71c | nd | 29.33 ± 0.71c | |
| Euphylla | 40.72 ± 1.01d | nd | 40.72 ± 1.01d | 29.28 ± 0.42c | nd | 29.28 ± 0.42c | |
| Syringic acid | Radicle | 13.19 ± 1.56a | nd | 13.19 ± 1.56a | 8.90 ± 0.15a | nd | 8.90 ± 0.15a |
| Germinal | 21.98 ± 3.40b | nd | 21.98 ± 3.40b | 11.84 ± 1.19b | 7.86 ± 0.03a | 19.70 ± 1.18b | |
| Fibril | 13.36 ± 1.06a | 9.85 ± 0.88a | 23.21 ± 1.86b | 12.51 ± 1.18b | 9.56 ± 0.15b | 22.07 ± 1.05c | |
| Euphylla | 15.67 ± 0.15a | 12.69 ± 0.17b | 28.36 ± 0.08c | 11.40 ± 0.57b | 10.74 ± 0.22c | 22.14 ± 0.76c | |
| Hydroxycinnamic acid | Radicle | 13.69 ± 0.25a | 60.73 ± 2.86a | 74.42 ± 2.87a | 13.12 ± 0.31a | 51.37 ± 0.68a | 64.49 ± 0.88a |
| Germinal | 17.15 ± 0.27b | 73.23 ± 7.19b | 90.38 ± 10.18b | 15.51 ± 0.18b | 58.84 ± 1.31b | 74.35 ± 1.49b | |
| Fibril | 18.26 ± 1.73b | 74.15 ± 4.43b | 92.41 ± 6.15b | 19.61 ± 0.15c | 72.13 ± 1.79c | 91.74 ± 1.92c | |
| Euphylla | 24.86 ± 0.36c | 109.3 ± 2.3c | 134.1 ± 2.6c | 21.31 ± 0.22d | 101.3 ± 2.9d | 122.6 ± 2.7d | |
| Ferulic acid | Radicle | 14.48 ± 0.37a | 290.5 ± 0.8a | 305.0 ± 0.5a | 13.99 ± 0.62a | 256.7 ± 9.5ab | 270.7 ± 9.9a |
| Germinal | 18.17 ± 0.05b | 342.0 ± 37.0b | 360.2 ± 37.0b | 15.96 ± 0.19b | 246.1 ± 7.8a | 262.1 ± 7.9a | |
| Fibril | 19.78 ± 1.83c | 312.1 ± 9.9ab | 331.9 ± 11.8ab | 20.04 ± 0.24c | 284.5 ± 5.6c | 304.5 ± 5.8b | |
| Euphylla | 27.30 ± 0.36d | 318.4 ± 6.0ab | 345.7 ± 5.7b | 22.12 ± 0.29d | 269.0 ± 11.5bc | 291.1 ± 11.2b | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, N.; Guo, X.; Liu, F.; Li, Q.; Hu, J.; Brennan, C.S. Effect of Light- and Dark-Germination on the Phenolic Biosynthesis, Phytochemical Profiles, and Antioxidant Activities in Sweet Corn (Zea mays L.) Sprouts. Int. J. Mol. Sci. 2017, 18, 1246. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061246
Xiang N, Guo X, Liu F, Li Q, Hu J, Brennan CS. Effect of Light- and Dark-Germination on the Phenolic Biosynthesis, Phytochemical Profiles, and Antioxidant Activities in Sweet Corn (Zea mays L.) Sprouts. International Journal of Molecular Sciences. 2017; 18(6):1246. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061246
Chicago/Turabian StyleXiang, Nan, Xinbo Guo, Fengyuan Liu, Quan Li, Jianguang Hu, and Charles Stephen Brennan. 2017. "Effect of Light- and Dark-Germination on the Phenolic Biosynthesis, Phytochemical Profiles, and Antioxidant Activities in Sweet Corn (Zea mays L.) Sprouts" International Journal of Molecular Sciences 18, no. 6: 1246. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18061246