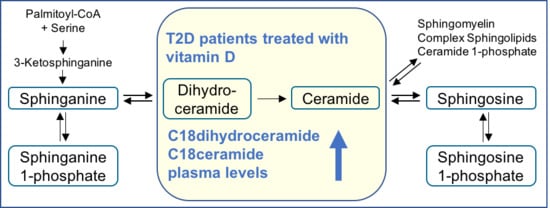
Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients
Abstract
:1. Introduction
2. Results
2.1. Clinical and Biochemical Parameters
2.2. Plasma Sphingolipid Levels
3. Discussion
4. Materials and Methods
4.1. Patients and Sampling
4.2. LC-MS/MS Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 25(OH)D3 | 25-hydroxyvitamin D3 |
| Cer | ceramide |
| CerS | ceramide synthase |
| dhCer | dihydroceramide |
| HDL | high densitiy lipoproteins |
| LDL | low densitiy lipoprotein |
| dhS1P | sphinganine 1-phosphate |
| S1P | sphingosine 1-phosphate |
| T2D | Type 2 Diabetes mellitus |
References
- Langer, J.; Penna-Martinez, M.; Bon, D.; Herrmann, E.; Wallasch, M.; Badenhoop, K. Insufficient vitamin D response to solar radiation in German patients with Type 2 Diabetes or gestational diabetes. Horm. Metab. Res. 2016, 48, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. Endocr. Soc. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Svobodová, J.; Imran, S.; Stanford, C.; Razzaque, M.S. Vitamin D deficiency: A single centre analysis of patients from 136 countries. J. Steroid Biochem. Mol. Biol. 2016, 164, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, B.; Mensink, G.B.; Thierfelder, W.; Muller, M.J.; Scheidt-Nave, C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Mattila, C.; Knekt, P.; Mannisto, S.; Rissanen, H.; Laaksonen, M.A.; Montonen, J.; Reunanen, A. Serum 25-hydroxyvitamin D concentration and subsequent risk of Type 2 Diabetes. Diabetes Care 2007, 30, 2569–2570. [Google Scholar] [CrossRef] [PubMed]
- Isaia, G.; Giorgino, R.; Adami, S. High prevalence of hypovitaminosis D in female Type 2 Diabetic population. Diabetes Care 2001, 24, 1496. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2010, 33, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Sabherwal, S.; Bravis, V.; Devendra, D. Effect of oral vitamin D and calcium replacement on glycaemic control in South Asian patients with Type 2 Diabetes. Int. J. Clin. Pract. 2010, 64, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Von Hurst, R.R.; Stonehouse, W.; Caad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, E.; Pittas, A.G. The role of vitamin D in the prevention of Type 2 Diabetes. To D or not to D? Endocrinology 2017, 158, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Galadari, A.; Thayyullathil, F. Role of ceramide in diabetes mellitus: Evidence and mechanisms. Lipids Health Dis. 2013, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, S.; Japtok, L.; Kleuser, B. Divergent role of sphingosine 1-phosphate on insulin resistance. Cell. Physiol. Biochem. 2014, 34, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Pfeilschifter, J.; Huwiler, A. Sphingosine 1-phosphate in renal diseases. Cell. Physiol. Biochem. 2013, 31, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bikman, B.T.; Summers, S.A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Investig. 2011, 121, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004, 53, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Baranowski, M.; Nikolajuk, A.; Otziomek, E.; Zabielski, P.; Adamska, A.; Blachnio, A.; Gorski, J.; Gorska, M. Increased skeletal muscle ceramide level in men at risk of developing Type 2 Diabetes. Diabetologia 2007, 50, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Skovbro, M.; Baranowski, M.; Skov-Jensen, C.; Flint, A.; Dela, F.; Gorski, J.; Helge, J.W. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 2008, 51, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Ceramide: A contributor to insulin resistance or an innocent bystander? Diabetologia 2008, 51, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Strobel, F.; Reusch, J.; Penna-Martinez, M.; Ramos-Lopez, E.; Klahold, E.; Klepzig, C.; Wehrle, J.; Kahles, H.; Badenhoop, K. Effect of a randomised controlled vitamin D trial on insulin resistance and glucose metabolism in patients with Type 2 Diabetes mellitus. Horm. Metab. Res. 2014, 46, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Eisman, J.A.; Bouillon, R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. BoneKEy Rep. 2014, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular endocrinology of vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Badenhoop, K. Genetics: Vitamin D and Type 2 Diabetes mellitus—Hype or hope? Nat. Rev. Endocrinol. 2015, 11, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M.; Pierce, J.S.; Soodavar, F.; Smith, K.J.; Al Gadban, M.M.; Rembiesa, B.; Klein, R.L.; Hannun, Y.A.; Bielawski, J.; Bielawska, A. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J. Lipid Res. 2010, 51, 3074–3087. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The Crucial Role of C18-Cer in Fat-Induced Skeletal Muscle Insulin Resistance. Cell. Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, O.; Futerman, A.H. The role of the ceramide acyl chain length in neurodegeneration: Involvement of ceramide synthases. Neuromol. Med. 2010, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Shimizu, Y.; Zhao, S.; Ukon, N.; Nishijima, K.; Wakabayashi, M.; Yoshioka, T.; Higashino, K.; Numata, Y.; Okuda, T.; et al. Characterization of the role of sphingomyelin synthase 2 in glucose metabolism in whole-body and peripheral tissues in mice. Biochim. Biophys. Acta 2016, 1861, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Brown, S.H.; Lim, X.Y.; Fiveash, C.E.; Osborne, B.; Bentley, N.L.; Braude, J.P.; Mitchell, T.W.; Coster, A.C.; Don, A.S.; et al. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: A beneficial role for very long-chain sphingolipid species. Biochim. Biophys. Acta 2016, 1861, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, W.J.; Kuperman, Y.; Boura-Halfon, S.; Pewzner-Jung, Y.; Futerman, A.H. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistancein mice due to altered detergent-resistant membranes. Hepatology 2013, 57, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with Type 2 Diabetes and correlates with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Véret, J.; Coant, N.; Berdyshev, E.V.; Skobeleva, A.; Therville, N.; Bailbé, D.; Gorshkova, I.; Natarajan, V.; Portha, B.; Le Stunff, H. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem. J. 2011, 438, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell. Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Mwinyi, J.; Boström, A.; Fehrer, I.; Othman, A.; Waeber, G.; Marti-Soler, H.; Vollenweider, P.; Marques-Vidal, P.; Schiöth, H.B.; von Eckardstein, A.; et al. Plasma 1-deoxysphingolipids are early predictors of incident Type 2 Diabetes mellitus. PLoS ONE 2017, 12, e0175776. [Google Scholar] [CrossRef]



| Patients’ Characteristics | Placebo | Verum | p-Value |
|---|---|---|---|
| Age (years) | 60 (52–66) | 62 (54–67) | 0.395 |
| BMI (kg/m2) | 31 (27–35) | 31 (27–33) | 0.791 |
| Duration of T2D (years) | 7 (4–10) | 5 (3–9) | 0.273 |
| Lipid Profile | Baseline | After 6 Months Supplementation | ||||
|---|---|---|---|---|---|---|
| Placebo | Verum | p-Value | Placebo | Verum | p-Value | |
| Triglycerides | 143 (104–222) | 151 (98–185) | 0.750 | 147 (111–198) | 145 (100–224) | 0.994 |
| Cholesterol | 194 (181–211) | 198 (174–227) | 0.738 | 200 (171–229) | 205 (176–231) | 0.733 |
| LDL-cholesterol | 109 (98–135) | 123 (97–144) | 0.275 | 118 (102–137) | 133 (96–145) | 0.524 |
| HDL-cholesterol | 46 (37–54) | 47 (37–58) | 0.837 | 48 (41–58) | 50 (40–56) | 0.727 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, A.; Grammatikos, G.; Trautmann, S.; Schreiber, Y.; Thomas, D.; Bruns, F.; Pfeilschifter, J.; Badenhoop, K.; Penna-Martinez, M. Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients. Int. J. Mol. Sci. 2017, 18, 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071532
Koch A, Grammatikos G, Trautmann S, Schreiber Y, Thomas D, Bruns F, Pfeilschifter J, Badenhoop K, Penna-Martinez M. Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients. International Journal of Molecular Sciences. 2017; 18(7):1532. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071532
Chicago/Turabian StyleKoch, Alexander, Georgios Grammatikos, Sandra Trautmann, Yannick Schreiber, Dominique Thomas, Franziska Bruns, Josef Pfeilschifter, Klaus Badenhoop, and Marissa Penna-Martinez. 2017. "Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients" International Journal of Molecular Sciences 18, no. 7: 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071532