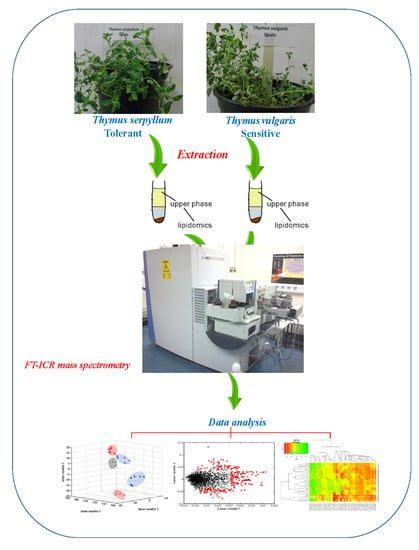
Lipidomics Unravels the Role of Leaf Lipids in Thyme Plant Response to Drought Stress
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lipid Profile Changes in Sensitive Thyme Plants under Drought Conditions
2.2. Lipid Profile Changes in Tolerant Thyme Plants under Drought Conditions
2.3. Integrative Analysis of Lipid Composition in Drought-Sensitive and -Tolerant Thyme Plants
2.3.1. Principal Component Analysis and Pattern Recognition Analysis of Mass Spectra
2.3.2. Outstanding Metabolites Involved in Plant Response Mechanism
2.4. Metabolic Pathway Analysis
3. Materials and Methods
3.1. Chemicals, Plant Materials, and Physiological Parameters
3.2. Tissue Harvesting and Sample Extraction
3.3. Lipidome Profiling by Direct Infusion Fourier Transform Ion Cyclotron Resonance (DI FT-ICR) Mass Spectrometry
3.4. Identification of the Extracted Metabolites
3.5. Chemometrics and Statistical Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DGDG | Digalactosyldiacylglycerol |
| DI FT-ICR | Direct infusion Fourier transform ion cyclotron resonance |
| FFA | Free fatty acid |
| KNN | K-nearest neighbor imputation method |
| MGDG | Monogalactosyldiacylglycerol |
| NL | Neutral lipids |
| PA | Phosphatidic acid |
| PC | Phosphatidylcholine |
| PCA | Principal component analysis |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PL | Phospholipids |
| PLS | Partial least square |
| PLSDA | Partial least square discriminant analysis |
| PQN | Probabilistic quotient normalization |
| PS | Phosphatidylserine |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| VIP | Variable importance on projection |
| 18:3, 18:2 | 18-Carbon chain PUFAs with three and two double bonds, respectively |
References
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E.; Sàez, F. Thyme: The Genus Thymtls; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Balentine, D.A.; Albano, M.C.; Nair, M.G. Role of medicinal plants, herbs, and spices in protecting human health. Nutr. Rev. 1999, 57, 41–45. [Google Scholar] [CrossRef]
- Caragay, A.B. Cancer-preventive foods and ingredients. Food Technol. 1992, 46, 65–68. [Google Scholar]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491s–499s. [Google Scholar] [PubMed]
- Zaika, L.L. Spices and herbs: Their antimicrobial activity and its determination1. J. Food Saf. 1988, 9, 97–118. [Google Scholar] [CrossRef]
- Aziz, A.; Larher, F. Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brassica napus L. J. Plant Physiol. 1998, 153, 754–762. [Google Scholar] [CrossRef]
- Dakhma, W.S.; Zarrouk, M.; Cherif, A. Effects of drought-stress on lipids in rape leaves. Phytochemistry 1995, 40, 1383–1386. [Google Scholar] [CrossRef]
- Svenningsson, H.; Liljenberg, C. Membrane lipid changes in root cells of rape (brassicanapus) as a function of water-deficit stress. Physiol. Plant. 1986, 68, 53–58. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Pinzino, C.; Sgherri, C.L.; Navari-Izzo, F. Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought. Plant Physiol. 1995, 108, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hubac, C.; Guerrier, D.; Ferran, J.; Tremolieres, A. Change of leaf lipid composition during water stress in two genotypes of lupinus albus resistant or susceptible to drought. Plant Physiol. Biochem. 1989, 27, 737–744. [Google Scholar]
- Bahl, J.; Francke, B.; Monéger, R. Lipid composition of envelopes, prolamellar bodies and other plastid membranes in etiolated, green and greening wheat leaves. Planta 1976, 129, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Eastman, P.; Rashid, A.; Camm, E. Changes of the photosystem 2 activity and thylakoid proteins in spruce seedlings during water stress. Photosynthetica 1998, 34, 201–210. [Google Scholar] [CrossRef]
- Pham, T.A.T.; Vieira da Silva, J.; Mazliak, P. The role of membrane lipids in drought resistance of plants. Bulletin de la Société Botanique de France. Actualités Botaniques 1990, 137, 99–114. [Google Scholar] [CrossRef]
- Kaoua, M.; Serraj, R.; Benichou, M.; Hsissou, D. Comparative sensitivity of two moroccan wheat varieties to water stress: The relationship between fatty acids and proline accumulation. Bot. Stud. 2006, 47, 51–60. [Google Scholar]
- El-Hafid, L.; Pham, T.A.; Zuily-Fodil, Y.; Vieira da Silva, J. Enzymatic Breakdown of Polar Lipids in Cotton Leaves under Water Stress. 1. Degradation of Monogalactosyl-Diacylglycerol. Plant Physiology Biochemistry 1989. Available online: http://agris.fao.org/agris-search/search.do?recordID=FR9001726 (accessed on 27 September 2017).
- Anh, T.P.T.; Borrel-Flood, C.; da Silva, J.V.; Justin, A.M.; Mazliak, P. Effects of water stress on lipid metabolism in cotton leaves. Phytochemistry 1985, 24, 723–727. [Google Scholar] [CrossRef]
- Hamrouni, I.; Salah, H.B.; Marzouk, B. Effects of water-deficit on lipids of safflower aerial parts. Phytochemistry 2001, 58, 277–280. [Google Scholar] [CrossRef]
- De Paula, F.M.; Thi, A.P.; de Silva, J.V.; Justin, A.; Demandre, C.; Mazliak, P. Effects of water stress on the molecular species composition of polar lipids from Vigna unguiculata L. Leaves. Plant Sci. 1990, 66, 185–193. [Google Scholar] [CrossRef]
- Repellin, A.; Thi, A.P.; Tashakorie, A.; Sahsah, Y.; Daniel, C.; Zuily-Fodil, Y. Leaf membrane lipids and drought tolerance in young coconut palms (Cocos nucifera L.). Eur. J. Agron. 1997, 6, 25–33. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.T. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Sabir, J.S.; Alakilli, S.Y.; Shokry, A.M.; Gadalla, N.O.; Edris, S.; Al-Korduy, M.A.; Al-Zahrani, H.S.; El-Domyati, F.M.; Bahieldin, A. Metabolomic response of calotropis procera growing in the desert to changes in water availability. PLoS ONE 2014, 9, e87895. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Saito, K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Moradi, P. Use of Metabolomics to Study Water Deficit Stress on the Medicinal Plant Thyme. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Perez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (pls-da) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar] [PubMed]
- Wold, S.; Trygg, J.; Berglund, A.; Antti, H. Some recent developments in pls modeling. Chemometr. Intell. Lab. Syst. 2001, 58, 131–150. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Key, M. A tutorial in displaying mass spectrometry-based proteomic data using heat maps. BMC Bioinform. 2012, 13, S10. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-Y.; Jung, H.-J.; Moon, M.H.; Chung, B.C.; Choi, M.H. Heat-map visualization of gas chromatography-mass spectrometry based quantitative signatures on steroid metabolism. J. Am. Soc. Mass Spectrom. 2009, 20, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-M.; Park, J.-Y.; Han, S.-H.; Anderson, A.J.; Yang, K.-Y.; Gardener, B.M.; Kim, Y.-C. Identification and transcriptional analysis of priming genes in arabidopsis thaliana induced by root colonization with pseudomonas chlororaphis o6. Plant Pathol. J. 2011, 27, 272–279. [Google Scholar] [CrossRef]
- Song, L.; Prince, S.; Valliyodan, B.; Joshi, T.; dos Santos, J.V.M.; Wang, J.; Lin, L.; Wan, J.; Wang, Y.; Xu, D. Genome-wide transcriptome analysis of soybean primary root under varying water-deficit conditions. BMC Genom. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- García-Calderon, M.; Pons-Ferrer, T.; Mrázova, A.; Pal‘ove-Balang, P.; Vilková, M.; Pérez-Delgado, C.M.; Vega, J.M.; Eliášová, A.; Repčák, M.; Márquez, A.J. Modulation of phenolic metabolism under stress conditions in a lotus japonicus mutant lacking plastidic glutamine synthetase. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakidara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K. Enhancement of oxidative and drought tolerance in arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, B.; Mostajeran, A.; Ghanadian, M. Flavonoid dynamic responses to different drought conditions: Amount, type, and localization of flavonols in roots and shoots of Arabidopsis thaliana L. Turk. J. Biol. 2016, 40, 612–622. [Google Scholar] [CrossRef]









| Pathway | Category | Coverage | p-Value | Database |
|---|---|---|---|---|
| α-Linolenic acid metabolism | Lipid metabolism | 0.33 | 0.0068255 | MI-Pack, MetaboAnalyst |
| Isoflavonoid biosynthesis | Biosynthesis of secondary metabolites | 0.25 | n/a | MI-Pack |
| Linoleic acid metabolism | Lipid metabolism | 0.23 | n/a | MI-Pack |
| Limonene and pinene degradations | Metabolism of terpenoids and polyketides | 0.2 | 0.021087 | MetaboAnalyst |
| Retinol metabolism | Metabolism of cofactors and vitamins | 0.13 | n/a | MI-Pack |
| Biosynthesis of unsaturated fatty acids | Lipid metabolism | 0.12 | 0.025999 | MetaboAnalyst |
| Steroid biosynthesis | Lipid metabolism | 0.1 | n/a | MI-Pack |
| Flavonoid biosynthesis | Biosynthesis of secondary metabolites | 0.1 | n/a | MI-Pack |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, P.; Mahdavi, A.; Khoshkam, M.; Iriti, M. Lipidomics Unravels the Role of Leaf Lipids in Thyme Plant Response to Drought Stress. Int. J. Mol. Sci. 2017, 18, 2067. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102067
Moradi P, Mahdavi A, Khoshkam M, Iriti M. Lipidomics Unravels the Role of Leaf Lipids in Thyme Plant Response to Drought Stress. International Journal of Molecular Sciences. 2017; 18(10):2067. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102067
Chicago/Turabian StyleMoradi, Parviz, Atiyeh Mahdavi, Maryam Khoshkam, and Marcello Iriti. 2017. "Lipidomics Unravels the Role of Leaf Lipids in Thyme Plant Response to Drought Stress" International Journal of Molecular Sciences 18, no. 10: 2067. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102067