The Contribution of Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma to the Treatment of Chronic Equine Laminitis: A Proof of Concept
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Characterization of Adipose Tissue-Derived MSCs
2.2. Quality Controls
2.3. Safety of Clinical Procedures
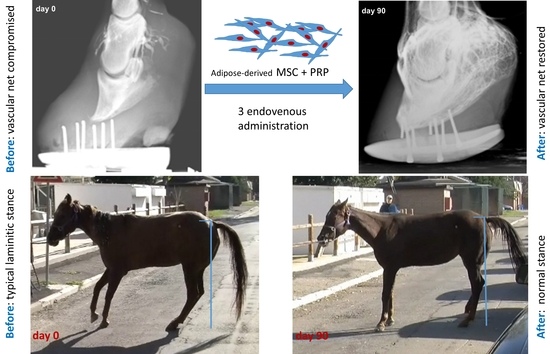
2.4. Efficacy and Outcome of the Therapy
3. Discussion
4. Materials and Methods
4.1. Stem Cell Isolation from Adipose Tissue
4.2. Platelet-Rich Plasma (PRP) Preparation
4.3. aMSC Preparation for Administration
4.4. Quality Controls
4.5. Selection and Description of Clinical Cases
4.6. aMSC Administration
4.7. Semi-Quantitative RT-PCR
5. Conclusions
Supplementary materials
Acknowledgments
Author contributions
Conflicts of interest
References
- Katz, L.M.; Bailey, S.R. A review of recent advances and current hypotheses on the pathogenesis of acute laminitis. Equine Vet. J. 2012, 44, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, C.C. The anatomy and physiology of the suspensory apparatus of the distal phalanx. Vet. Clin. North Am. Equine Pract. 2010, 26, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Engiles, J.B.; Galantino-Homer, H.L.; Boston, R.; McDonald, D.; Dishowitz, M.; Hankenson, K.D. Osteopathology in the equine distal phalanx associated with the development and progression of laminitis. Vet. Pathol. 2015, 52, 928–944. [Google Scholar] [CrossRef] [PubMed]
- Eades, S.C. Overview of current laminitis research. Vet. Clin. North Am. Equine Pract. 2010, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Keen, J.; McGowan, C. Equine metabolic syndrome. Vet. Rec. 2015, 177, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Eustace, R.A. Clinical presentation, diagnosis, and prognosis of chronic laminitis in Europe. Vet. Clin. North Am. Equine Pract. 2010, 26, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, C.; Collins, S.N. Chronic laminitis. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders Health Sciences: St. Louis, MO, USA, 2010; pp. 374–377. [Google Scholar]
- Bailey, S.R.; Marr, C.M.; Elliott, J. Current research and theories on the pathogenesis of acute laminitis in the horse. Vet. J. 2004, 167, 129–142. [Google Scholar] [CrossRef]
- Pollitt, C. Pathophysiology of laminitis. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders Health Sciences: St. Louis, MO, USA, 2010; pp. 366–371. [Google Scholar]
- Black, S.J. Extracellular matrix, leukocyte migration and laminitis. Vet. Immunol. Immunopathol. 2009, 129, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Steelman, S.M.; Johnson, D.; Wagner, B.; Stokes, A.; Chowdhary, B.P. Cellular and humoral immunity in chronic equine laminitis. Vet. Immunol. Immunopathol. 2013, 153, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Belknap, J.K.; Faleiros, R.; Black, S.J.; Johnson, P.J.; Eades, S. The laminar leukocyte: From sepsis to endocrinopathic models of laminitis. J. Vet. Sci. 2011, 31, 584–585. [Google Scholar] [CrossRef]
- Loftus, J.P.; Johnson, P.J.; Belknap, J.K.; Pettigrew, A.; Black, S.J. Leukocyte-derived and endogenous matrix metalloproteinases in the lamellae of horses with naturally acquired and experimentally induced laminitis. Vet. Immunol. Immunopathol. 2009, 129, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Pettigrew, A.; Cochran, A.M.; Belknap, J.K. Indices of inflammation in the lung and liver in the early stages of the black walnut extract model of equine laminitis. Vet. Immunol. Immunopathol. 2009, 129, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Tadros, E.M.; Frank, N.; Newkirk, K.M.; Donnell, R.L.; Horohov, D.W. Effects of a “two-hit” model of organ damage on the systemic inflammatory response and development of laminitis in horses. Vet. Immunol. Immunopathol. 2012, 150, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Belknap, J.K.; Moore, J.N.; Crouser, E.C. Sepsis-From human organ failure to laminar failure. Vet. Immunol. Immunopathol. 2009, 129, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Geor, R.; Frank, N. Metabolic syndrome-From human organ disease to laminar failure in equids. Vet. Immunol. Immunopathol. 2009, 129, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Liu, J.; Lee, J.W. Cell-based therapy for acute organ injury: Preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014, 121, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Pati, S.; Lee, J.W. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells 2017, 35, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.; Bakar, E.; Cerkezkayabekir, A.; Sanal, F.; Ulucam, E.; Subaşı, C.; Karaöz, E. Mesenchymal stem cells increase antioxidant capacity in intestinal ischemia/reperfusion damage. J. Pediatr. Surg. 2017, 52, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hong, X.; Potter, C.; Qu, A.; Xu, Q. Mesenchymal stem cells and vascular regeneration. Microcirculation 2017. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Tajima, S.; Mizuno, H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: Stem cell transplantation methods that enhance stemness. Stem Cell Res. Ther. 2015, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton’s jelly in the horse. Reproduction 2012, 143, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vázquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H. Update on the mechanisms of homing of adipose tissue-derived stem cells. Cytotherapy 2016, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Block, G.J.; Ohkouchi, S.; Fung, F.; Frenkel, J.; Gregory, C.; Pochampally, R.; DiMattia, G.; Sullivan, D.E.; Prockop, D.J. Multipotent stromal cells (MSCs) are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1 (STC-1). Stem Cells 2009, 27, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ohkouchi, S.; Kanehira, M.; Tode, N.; Kobayashi, M.; Ebina, M.; Nukiwa, T.; Irokawa, T.; Ogawa, H.; Akaike, T.; et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. 2015, 23, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Lin, H.C.; Huang, Y.H.; Hung, S.C. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J. Hepatol. 2015, 63, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Yu, J.M.; Foskett, A.M.; Peltier, G.; Reneau, J.C.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16766–16771. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, B.; Kuang, D.; Song, G. Role of Stromal-Derived Factor-1 in Mesenchymal Stem Cell Paracrine-Mediated Tissue Repair. Curr. Stem Cell Res. Ther. 2016, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M. The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv. Wound Care 2015, 4, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liu, Z.; Li, X.; Zhang, B.; Wang, X.; Lan, J.; Shi, Q.; Li, D.; Ju, X. Migration ability and Toll-like receptor expression of human mesenchymal stem cells improves significantly after three-dimensional culture. Biochem. Biophys. Res. Commun. 2017, 491, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Alessandri, G.; Dall’Aglio, C.; Mercati, F.; Coliolo, P.; Bazzucchi, C.; Dante, S.; Petrini, S.; Curina, G.; Ceccarelli, P. Membrane vesicles mediate pro-angiogenic activity of equine adipose-derived mesenchymal stromal cells. Vet. J. 2014, 202, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Concise Review: The Use of Adipose-Derived Stromal Vascular Fraction Cells and Platelet Rich Plasma in Regenerative Plastic Surgery. Stem Cells 2017, 35, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Riccò, S.; Renzi, S.; Del Bue, M.; Conti, V.; Merli, E.; Ramoni, R.; Lucarelli, E.; Gnudi, G.; Ferrari, M.; Grolli, S. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int. J. Immunopathol. Pharmacol. 2013, 26, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.; Zimmerman, M.; Crocetti, S.; Suls, M.; Mariën, T.; Ferguson, S.J.; Chiers, K.; Duchateau, L.; Franco-Obregón, A.; Wuertz, K.; et al. Regenerative therapies for equine degenerative joint disease: A preliminary study. PLoS ONE 2014, 9, e85917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canapp, S.O.; Canapp, D.A.; Ibrahim, V.; Carr, B.J.; Cox, C.; Barrett, J.G. The Use of Adipose-Derived Progenitor Cells and Platelet-Rich Plasma Combination for the Treatment of Supraspinatus Tendinopathy in 55 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Carrade, D.D.; Borjesson, D.L. Immunomodulation by mesenchymal stem cells in veterinary species. Comp. Med. 2013, 63, 207–217. [Google Scholar] [PubMed]
- Kol, A.; Wood, J.A.; Carrade Holt, D.D.; Gillette, J.A.; Bohannon-Worsley, L.K.; Puchalski, S.M.; Walker, N.J.; Clark, K.C.; Watson, J.L.; Borjesson, D.L. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res. Ther. 2015, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Ardanaz, N.; Vázquez, F.J.; Romero, A.; Remacha, A.R.; Barrachina, L.; Sanz, A.; Ranera, B.; Vitoria, A.; Albareda, J.; Prades, M.; et al. Inflammatory response to the administration of mesenchymal stem cells in an equine experimental model: Effect of autologous, and single and repeat doses of pooled allogeneic cells in healthy joints. BMC Vet. Res. 2016, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.D.; Kol, A.; Walker, N.J.; Borjesson, D.L. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int. 2016, 2016, 5830103. [Google Scholar] [CrossRef] [PubMed]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 2015, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Sole, A.; Spriet, M.; Galuppo, L.D.; Padgett, K.A.; Borjesson, D.L.; Wisner, E.R.; Vidal, M.A. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using (99m) Tc-HMPAO. Equine Vet. J. 2012, 44, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, G.I.; Pollitt, C.C. Progression of venographic changes after experimentally induced laminitis. Vet. Clin. North Am. Equine Pract. 2010, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- De Mattos Carvalho, A.; Alves, A.L.; Golim, M.A.; Moroz, A.; Hussni, C.A.; de Oliveira, P.G.; Deffune, E. Isolation and immunophenotypic characterization of mesenchymal stem cells derived from equine species adipose tissue. Vet. Immunol. Immunopathol. 2009, 132, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Barberini, D.J.; Freitas, N.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; da Cruz Landim-Alvarenga, F.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Floyd, A.E. Grading the laminitic horse. In Equine Podiatry; Floyd, A.E., Mansmann, R.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2007; pp. 320–327. [Google Scholar]
- D’Arpe, L.; Bernardini, D. Digital venography in horses and its clinical application in Europe. Vet. Clin. North Am. Equine Pract. 2010, 26, 339–359. [Google Scholar] [CrossRef] [PubMed]


| Marker Gene | Expression in Equine aMSCs | % Target Gene vs β actin Gene Expression |
|---|---|---|
| CD44 | positive | +++ |
| CD29 | positive | +++ |
| CD90 | positive | +++ |
| CD45 | negative | − |
| CD34 | negative | − |
| CD73 | positive | ++ |
| CD13 | positive | +++ |
| CD133 | positive | ++ |
| CD105 | positive | ++ |
| CD31 | negative | − |
| Oct-4 | positive | + |
| IL1-Ra | positive | ++ |
| SDF-1 | positive | ++ |
| CXCR4 | − | − |
| TSG-6 | positive | +++ |
| STC-1 | positive | ++ |
| Patient | Breed | Age | Sex | Activity | 6-Month Outcome | 1-Year Outcome | >1 Year Outcome (1–3 Years) |
|---|---|---|---|---|---|---|---|
| CASE 1 | QUARTER HORSE | 18 | F | MARE | returned to activity | still in activity | deceased (intestinal colic) |
| CASE 2 | QUARTER HORSE | 14 | F | MARE | returned to activity | still in activity | deceased |
| CASE 3 | THOROUGHBRED | 13 | G | PLEASURE | returned to activity | still in activity | still in activity |
| CASE 4 | QUARTER HORSE | 10 | F | PLEASURE | returned to activity | still in activity | still in activity |
| CASE 5 | WARMBLOOD | 18 | G | PLEASURE | returned to activity | still in activity | deceased (intestinal colic) |
| CASE 6 | WARMBLOOD | 15 | F | PLEASURE | returned to activity | recurrence of laminitis | euthanized |
| CASE 7 | DUTCH WARMBLOOD | 21 | F | PLEASURE | returned to activity | recurrence of laminitis | euthanized |
| CASE 8 | THOROUGHBRED | 15 | G | PLEASURE | returned to activity | still in activity | not applicable |
| CASE 9 | PONY | 16 | G | PLEASURE | returned to activity | still in activity | not applicable |
| Patient | Breed | Age | Sex | Activity | Laminitis Stage | Venography Stage | Prognosis (before Treatment) |
|---|---|---|---|---|---|---|---|
| CASE 1 | QUARTER HORSE | 18 | F | MARE | BILATERAL ROTATION | 4 | POOR |
| CASE 2 | QUARTER HORSE | 14 | F | MARE | BILATERAL CHRONIC ROTATION | 4 | POOR |
| CASE 3 | THOROUGHBRED | 13 | G | PLEASURE | MONOLATERAL SINKING AND ROTATION | 4 | POOR |
| CASE 4 | QUARTER HORSE | 10 | F | PLEASURE | MONOLATERAL SINKING | 3 | GUARDED PROGNOSIS |
| CASE 5 | WARMBLOOD | 18 | G | PLEASURE | BILATERAL SINKING AND ROTATION | 3 | POOR |
| CASE 6 | WARMBLOOD | 15 | F | PLEASURE | MONOLATERAL SINKING AND ROTATION | 3 | POOR |
| CASE 7 | DUTCH WARMBLOOD | 21 | F | PLEASURE | BILATERAL SINKING AND ROTATION | 3-4 | POOR |
| CASE 8 | THOROUGHBRED | 15 | G | PLEASURE | MONOLATERAL ROTATION | 3 | POOR |
| CASE 9 | PONY | 16 | G | PLEASURE | BILATERAL SINKING AND ROTATION | 4 | POOR |
| Marker | Accession Number | pRIMER SEQUENCE | AMPLICON SIZE |
|---|---|---|---|
| β-ACTIN | AF035774.1 | Fw: ACCCCGTGCTGCTGACCGA Rv: GCAGAAGGAGATCACAGCCCT | 658 bp |
| CD44 | X66862.1 | Fw: CAGACCTGCCCAACGCCTTCGAGGGAC Rv: CAGAGCCAGGGCCAGGAGGGACGCC | 440 bp |
| CD29 | NM_001301217.1 | Fw: ACAGATGCCGGGTTTCACTTTGC Rv: CCATTTTCCCCTGTTCCCATTCACCC | 405 bp |
| CD90 | EU881920 | Fw: ATCGCTCTCCTGCTGACAGT Rv: CGGAGTTCGCATGTGTAGAG | 302 bp |
| CD45 | XM_008543985.1 | Fw: TCCATGCAGATATTTTGTTGGACAC Rv: ATTGATGGCCAGTATTCTGCACACTTG | 409 bp |
| CD34 | XM_005609830.2 | Fw: ATGCTGGTCCGCAGGGGCGCGCGC Rv: GGGCAAGGAGCAAGGAGCACAC | 684 bp |
| CD73 | XM_001500115 | Fw: CAAAAAGGCCAACTTTCCAA Rv: AACCTTCCGTCCATCATCAG | 430 bp |
| CD13 | NM_001150 | Fw: GGCAGATGACCTGGCGGGC Rv: ACCACCCGCTCCTTGTTG | 591 bp |
| CD133 | XM_001498679 | Fw: TGTGTGGGACTACCGTTTCA Rv: TAGGTGGTGATTTGCCACAA | 512 bp |
| CD105 | XM_001500078 | Fw: GCTGACGACAGAGATGACCA Rv: TCCTGGGATACAGGGCTATG | 484 bp |
| CD31 | NM_00110165 | Fw: AAAGGGCCCAATACATTT Rv: GCAGGTATAGTGCCCGCTGT | 686 bp |
| Oct-4 | XM_001490108.2 | Fw: TCCCAGGACATCAAAGCTCAGA Rv: TCTGGGCTCTCCCATGCATTCAAAC | 321 bp |
| IL1-Ra | XM_005599766.2 | Fw: ATGGAAATCCGCAGGCGTTCTG Rv: CTACTGGTCCTCCTGGAGGTAG | 531 bp |
| SDF-1 | KF612401.1 | Fw: ATGAACGCCAAGGTCGTCGCCG Rv: CTTGTTTAAAGCTTTCTCCAGGTAC | 359 bp |
| CXCR4 | XM_001490165 | Fw: CTACACAGTCAACCTCTCCAGC Rv: CTGCTCACAGAGGTGAGTGCATGC | 597 bp |
| TSG-6 | NM_001081906.1 | Fw: ATGATCATCTTAATTTACGTACTTG Rv: TTATAAATGGGAAAACTTGG | 845 bp |
| STC-1 | XM_001493195.4 | Fw: TGATCAGTGCTTCTGCAACC Rv: TCACAGTCCAGTAGGCTTCG | 466 bp |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelone, M.; Conti, V.; Biacca, C.; Battaglia, B.; Pecorari, L.; Piana, F.; Gnudi, G.; Leonardi, F.; Ramoni, R.; Basini, G.; et al. The Contribution of Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma to the Treatment of Chronic Equine Laminitis: A Proof of Concept. Int. J. Mol. Sci. 2017, 18, 2122. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102122
Angelone M, Conti V, Biacca C, Battaglia B, Pecorari L, Piana F, Gnudi G, Leonardi F, Ramoni R, Basini G, et al. The Contribution of Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma to the Treatment of Chronic Equine Laminitis: A Proof of Concept. International Journal of Molecular Sciences. 2017; 18(10):2122. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102122
Chicago/Turabian StyleAngelone, Mario, Virna Conti, Cristiano Biacca, Beatrice Battaglia, Laura Pecorari, Francesco Piana, Giacomo Gnudi, Fabio Leonardi, Roberto Ramoni, Giuseppina Basini, and et al. 2017. "The Contribution of Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma to the Treatment of Chronic Equine Laminitis: A Proof of Concept" International Journal of Molecular Sciences 18, no. 10: 2122. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18102122