Adjuvant Biological Therapies in Chronic Leg Ulcers
Abstract
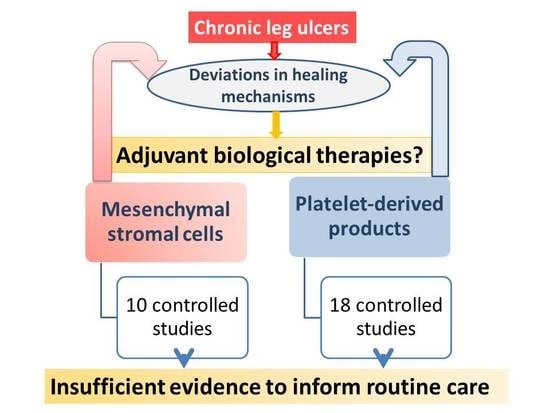
:1. Introduction
2. Results
2.1. Cell-Based Studies
2.2. Platelet-Based Therapies
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Selection Criteria
4.3. Data Extraction
4.4. Risk of Bias Assessment
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Driver, V.R.; Fabbi, M.; Lavery, L.A.; Gibbons, G. The costs of diabetic foot: The economic case for the limb salvage team. J. Vasc. Surg. 2010, 52, 17s–22s. [Google Scholar] [CrossRef] [PubMed]
- Fife, C.E.; Carter, M.J. Wound care outcomes and associated cost among patients treated in us outpatient wound centers: Data from the US wound registry. Wounds Compend. Clin. Res. Pract. 2012, 24, 10–17. [Google Scholar]
- Global Industry Analysis, I. Advances Wound Care—A Global Strategies Bussines Report. Available online: http://www.strategyr.com/Advanced_Wound_Care_Market_Report.asp#sthash.FTA2Umdo.mqm1lRio.dpbs (accessed on 4 October 2017).
- Fife, C.; Walker, D.; Thomson, B.; Carter, M. Limitations of daily living activities in patients with venous stasis ulcers undergoing compression bandaging: Problems with the concept of self-bandaging. Wounds Compend. Clin. Res. Pract. 2007, 19, 255–257. [Google Scholar]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.C.; Bevilacqua, N.J.; Armstrong, D.G. The use of marrow-derived stem cells to accelerate healing in chronic wounds. Int. Wound J. 2008, 5, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Waycaster, C.; Schaum, K.; Gilligan, A.M. Cost-effectiveness of three adjunct cellular/tissue-derived products used in the management of chronic venous leg ulcers. Value Health 2014, 17, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montequin, J.I.; Valenzuela-Silva, C.M.; Diaz, O.G.; Savigne, W.; Sancho-Soutelo, N.; Rivero-Fernandez, F.; Sanchez-Penton, P.; Morejon-Vega, L.; Artaza-Sanz, H.; Garcia-Herrera, A.; et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: Multicenter, randomised, placebo-controlled, double-blind study. Int. Wound J. 2009, 6, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, Y.; Matsumoto, T.; Itoh, H.; Okazaki, J.; Uchiyama, M.; Yoshida, K.; Onimaru, M.; Onohara, T.; Inoguchi, H.; Kyuragi, R.; et al. DVC1–0101 to treat peripheral arterial disease: A phase I/IIa open-label dose-escalation clinical trial. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Senet, P.; Vicaut, E.; Beneton, N.; Debure, C.; Lok, C.; Chosidow, O. Topical treatment of hypertensive leg ulcers with platelet-derived growth factor-bb: A randomized controlled trial. Arch. Dermatol. 2011, 147, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Biological therapies in regenerative sports medicine. Sports Med. 2017, 47, 807–828. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Kim, H.R.; Kim, W.K. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: A pilot study. Wound Repair Regen. 2010, 18, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Perakath, B.; Jesudason, M.R.; Nayak, S. The effect of autologous bone marrow-derived cells on healing chronic lower extremity wounds: Results of a randomized controlled study. Ostomy Wound Manag. 2011, 57, 38–44. [Google Scholar]
- Kirana, S.; Stratmann, B.; Prante, C.; Prohaska, W.; Koerperich, H.; Lammers, D.; Gastens, M.H.; Quast, T.; Negrean, M.; Stirban, O.A.; et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int. J. Clin. Pract. 2012, 66, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, V.; Gumulec, J.; Jaluvka, F.; Salounova, D.; Jonszta, T.; Czerny, D.; Krajca, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Bertozzi, N.; Bonomini, S.; Bernuzzi, G.; Formentini, A.; Grignaffini, E.; Pio Grieco, M. Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy. Wounds Compend. Clin. Res. Pract. 2016, 28, 126–131. [Google Scholar]
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schluter, M.; Tonn, T.; Seeger, F.; Dimmeler, S.; Lindhoff-Last, E.; et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA). Circ. Cardiovasc. Interv. 2011, 4, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Aguirre, J.J.; Algorta, J.; Ayerdi, E.; Cabezas, A.I.; Orive, G.; Andia, I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.; Jorgensen, B.; Karlsmark, T.; Jorgensen, L.N.; Agren, M.S. Effect of topical autologous platelet-rich fibrin versus no intervention on epithelialization of donor sites and meshed split-thickness skin autografts: A randomized clinical trial. Plast. Reconstr. Surg. 2008, 122, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Driver, V.R.; Hanft, J.; Fylling, C.P.; Beriou, J.M. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manag. 2006, 52, 68–70. [Google Scholar]
- Jeong, S.H.; Han, S.K.; Kim, W.K. Treatment of diabetic foot ulcers using a blood bank platelet concentrate. Plast. Reconstr. Surg. 2010, 125, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kakagia, D.D.; Kazakos, K.J.; Xarchas, K.C.; Karanikas, M.; Georgiadis, G.S.; Tripsiannis, G.; Manolas, C. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J. Diabetes Complicat. 2007, 21, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Afshar, M.; Salimian, M.; Sharif, A.; Hidariyan, M. The effect of platelet rich plasma dressing on healing diabetic foot ulcers. Nurs. Midwifery Stud. 2016, 5. [Google Scholar] [CrossRef]
- Knighton, D.R.; Ciresi, K.; Fiegel, V.D.; Schumerth, S.; Butler, E.; Cerra, F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg. Gynecol. Obstet. 1990, 170, 56–60. [Google Scholar] [PubMed]
- Krupski, W.C.; Reilly, L.M.; Perez, S.; Moss, K.M.; Crombleholme, P.A.; Rapp, J.H. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: A preliminary report. J. Vasc. Surg. 1991, 14, 526–532. [Google Scholar] [CrossRef]
- Li, L.; Chen, D.; Wang, C.; Yuan, N.; Wang, Y.; He, L.; Yang, Y.; Chen, L.; Liu, G.; Li, X.; et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015, 23, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Moneib, H.A.; Youssef, S.S.; Aly, D.G.; Rizk, M.A.; Abdelhakeem, Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Obolenskiy, V.N.; Ermolova, D.A.; Laberko, L.A. Clinical and economic effectiveness of the use of platelet-rich plasma in the treatment of chronic wounds. Wound Med. 2017, 19, 27–32. [Google Scholar] [CrossRef]
- Pravin, A.J.S.; Sridhar, V.; Srinivasan, B.N. Autologous platelet rich plasma (PRP) versus leucocyte-platelet rich fibrin (L-PRF) in chronic non-healing leg ulcers—A randomised, open labelled, comparative study. J. Evol. Med. Dent. Sci. 2016, 5, 7460. [Google Scholar] [CrossRef] [PubMed]
- Saad Setta, H.; Elshahat, A.; Elsherbiny, K.; Massoud, K.; Safe, I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int. Wound J. 2011, 8, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Saldalamacchia, G.; Lapice, E.; Cuomo, V.; De Feo, E.; D’Agostino, E.; Rivellese, A.A.; Vaccaro, O. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 395–396. [Google Scholar] [CrossRef]
- Senet, P.; Bon, F.X.; Benbunan, M.; Bussel, A.; Traineau, R.; Calvo, F.; Dubertret, L.; Dosquet, C. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J. Vasc. Surg. 2003, 38, 1342–1348. [Google Scholar] [CrossRef]
- Stacey, M.C.; Mata, S.D.; Trengove, N.J.; Mather, C.A. Randomised double-blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2000, 20, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Steed, D.L.; Goslen, J.B.; Holloway, G.A.; Malone, J.M.; Bunt, T.J.; Webster, M.W. Randomized prospective double-blind trial in healing chronic diabetic foot ulcers. Ct-102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992, 15, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Roh, S.G.; Sharaf, B.; Lee, N.H. Risk of major limb amputation in diabetic foot ulcer and accompanying disease: A meta-analysis. J. Plast. Reconstruct. Aesthet. Surg. 2017, 70, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Agostini, C.; Avogaro, A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010, 209, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Teraa, M.; Sprengers, R.W.; van der Graaf, Y.; Peters, C.E.; Moll, F.L.; Verhaar, M.C. Autologous bone marrow-derived cell therapy in patients with critical limb ischemia: A meta-analysis of randomized controlled clinical trials. Ann. Surg. 2013, 258, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Catherine Foss, S.R.; Ambrose, J.; Carney, J.; Nilsson, M. International Federation for Adipose Therapeutics and Science. Available online: http://www.ifats.org/ (accessed on 4 October 2017).
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Dimarino, A.M.; Caplan, A.I.; Bonfield, T.L. Mesenchymal stem cells in tissue repair. Front. Immunol. 2013, 4, 201. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.J.; Al-Sayegh, H.; Freeman, M.D.; Smith, J.; Murrell, W.D.; Bubnov, R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int. Orthop. 2016, 40, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Chang, J.J.; Lee, J.H.; Lee, S.H. Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet. Disord. 2013, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Peeters, C.M.; Leijs, M.J.; Reijman, M.; van Osch, G.J.; Bos, P.K. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: A systematic literature review. Osteoarthr. Cartil. 2013, 21, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Knighton, D.R.; Ciresi, K.F.; Fiegel, V.D.; Austin, L.L.; Butler, E.L. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann. Surg. 1986, 204, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma: Combinational treatment modalities for musculoskeletal conditions. Front. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Muscle and tendon injuries: The role of biological interventions to promote and assist healing and recovery. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2015, 31, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; Zalduendo, M.M.; de la Fuente, M.; Prado, R.; Orive, G.; Andia, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty 2011, 11, e38. [Google Scholar] [PubMed]
- Martinez-Zapata, M.J.; Marti-Carvajal, A.J.; Sola, I.; Exposito, J.A.; Bolibar, I.; Rodriguez, L.; Garcia, J.; Zaror, C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum information for studies evaluating biologics in orthopaedics (MIBO): Platelet-rich plasma and mesenchymal stem cells. J. Bone Jt. Surg. Am. Vol. 2017, 99, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.P.; Li, W.W.; de Leon, J.; Driver, V.R.; Serena, T.E.; Wilson, J. Analysis of run-in and treatment data in a wound outcomes registry: Clinical impact of topical platelet-rich plasma gel on healing trajectory. Int. Wound J. 2011, 8, 638–650. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.M.; Driver, V.R.; Fylling, C.P.; Carter, M.J.; Anderson, C.; Wilson, J.; Dougherty, R.M.; Fuston, D.; Trigilia, D.; Valenski, V.; et al. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv. Skin Wound Care 2011, 24, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Kantor, J.; Santanna, J.; Strom, B.L.; Berlin, J.A. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001, 24, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Futrega, K.; King, M.; Lott, W.B.; Doran, M.R. Treating the whole not the hole: Necessary coupling of technologies for diabetic foot ulcer treatment. Trends Mol. Med. 2014, 20, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhao, K.; Liu, H.L.; Zhao, H.M.; Yang, J.; Sun, X.K. Infected bone inactivation combined with transplantation of autologous platelet-rich plasma and bone marrow for treatment of chronic osteomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4488–4493. [Google Scholar] [PubMed]
- Morimoto, N.; Kakudo, N.; Matsui, M.; Ogura, T.; Hara, T.; Suzuki, K.; Yamamoto, M.; Tabata, Y.; Kusumoto, K. Exploratory clinical trial of combination wound therapy with a gelatin sheet and platelet-rich plasma in patients with chronic skin ulcers: Study protocol. BMJ Open 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zabala, E.; Basterretxea, A.; Larrazabal, A.; Perez-Del-Pecho, K.; Rubio-Azpeitia, E.; Andia, I. Biological approach for the management of non-healing diabetic foot ulcers. J. Tissue Viability 2016, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Available online: www.cochrane-handbook.org (accessed on 9 October 2017).
- PRISMA. Preferred reporting items for systematic reviews and meta-analysis. Syst. Rev. 2015, 4, 1. [Google Scholar]
- MEDLINE. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pubmed (accessed on 30 July 2017).
- EMBASE. Available online: http://0-ovidsp-uk-ovid-com.brum.beds.ac.uk/sp-3.27.1a/ovidweb.cgi (accessed on 30 July 2017).
- The Cochrane Library Clinical Trials Database. Available online: http://www.cochranelibrary.com/ (accessed on 30 July 2017).



| Reference (Year) | Study Design Experimental/Control Group | Patient Population | Selection Criteria | Biological Intervention and Control Management | Outcomes/Follow-up | Differences and Statistical Results |
|---|---|---|---|---|---|---|
| Dash (2009) [13] | RCT Exp N = 12 CTR N = 12 | Diabetic foot and Burger disease | Chronicity > 1 month | Exp: BM-MSCs CD90+, CD105+, CD34−, expanded for 5 passages or more CTR: standard wound dressing | Pain-free walking distance and reduction in ulcer size/12 weeks | Cell implant group better than control in pain-free walking and reduction in ulcer area |
| Dubsky (2013) [14] | Consecutive patients (non-randomized) Three armed Exp1, N = 17 patients; Exp2, N = 11 patients CTR N = 22 | Diabetic foot disease | Critical limb ischemia PEDIS 3 TcPO2 30 mmHG or ABI < 0.6 | Exp1: BMC Exp2: PBMNC (after G-CSF mobilization) CTR: standard care | Rate of major amputation and TcO2/6 months Lost to follow-up: 3 | Amputations: 11% in the SCT vs. 50% CTR (p = 0.0032) No differences between BMC and PBMNC |
| Han (2009) [15] | RCT Exp N = 26 patients CTR N = 26 | Diabetic ulcers | Chronicity > 6 weeks | Exp1: SVF+ (fibrinogen/fibrin) C: (fibrinogen/fibrin) | Ulcer size/8 weeks Lost to follow-up: 2 | 100% complete wound healing in intervention and 62% complete healing in control group |
| Jain (2011) [16] | RCT Exp, N = 25 patients CTR N = 23 | Chronic lower limb foot in patients with diabetes mellitus | Chronicity > 3 months | Exp: BMC injection CTR: peripheral blood injection | Complete closure Area reduction Wound suitable for surgery | 40% ulcer healed in Exp vs. 29% in CTR p < 0.05 Area reduction: Exp: 36%/SD0.48 CTR: 27.32% SD0.32 No differences between groups at 3 months Exp N = 3 vs. CTR N = 1 had skin grafts/3 months Lost to follow-up: 2 |
| Kirana (2012) [17] | RCT Exp 1, N = 12 patients Exp 2 N = 12 CTR N = 6 | Diabetic ulcers | Chronicity > 6 weeks | Exp1: BMC Exp2: TRC/BM-MSC expanded enriched in CD90+ cells CTR: high n° of drop outs, 4/6 led to exclusion | Complete healing/8 week. Lost to follow-up: 2 Secondary endpoints: time to complete healing, n° major amputation improvement in ABI, TcPO2, BOLD | 22 patients received cell treatment. One patient in the TRC group and two in the BMC group did not show wound healing during follow up, 18 patients healed |
| Lu (2011) [18] | RCT, three armed-study Exp1, N = 20 patients; Exp2, N = 21 patients CTR: contra-lateral ulcer, N = 41 | Diabetic patients with CLI and foot ulcer | Bilateral critical limb ischemia (ABI 0.30–0.60) | Exp1: expanded BM-MSC with autologous serum Exp2: BMC CTR: normal saline | Ulcer healing rate, pain at rest and at walking, ABI, TcO2, MRA/24 weeks Lost to follow-up: 4 | BM-MSC better than BMC in pain at walking (p = 0.040), ABI p = 0.017, TcO2 p = 0.001, MRA p = 0.018 Cell treated ulcers better than controls in all outcome measures. After 6 weeks the number of healing ulcers in Exp1 was significantly higher than Exp2 |
| Marino (2013) [19] | Cohort study Exp N = 10 CTRL N = 10 | Arteriopathic patients, 18/20 had Diabetes mellitus type 2, five had heart disease and 6 had chronic obstructive pulmonary disease | ABI = 0.3–0.4. all patients underwent revascularization procedure without healing and hyperbaric chamber and oxygen therapy for 6 months | Exp1: SVF, Celution system® (5 mL) cells injected, in all directions, at the edge of the ulcer, depth 1 cm CTR: SVF untreated | Complete closure (primary) Decrease in diameter and depth (secondary) | Follow-up: 4, 10, 20, 60 and 90 days Complete healing in six of 10 patients Four patients did not respond to SVF treatment |
| Procházka (2010) [20] | RCT Exp N = 42 CTR N = 54 | 96 patients with diabetes except 5 in the experimental group; all with CLI and foot ulcer | chronic and critical limb ischemia according to the TASC classification Rutherford 4–6, Fontaine IV | Exp: BMC injection CTR: conventional treatment 40 injections each 1 mL into the ischemic limb | Major limb amputation during 120 days/13 patients died of causes unrelated to therapy | Amputation rate Exp: 21% CTR: 44% |
| Raposio (2016) [21] | RCT Exp, N = 16 patients (21 ulcers) CTR, N = 24 patients (31 ulcers) | Chronic skin ulcers (diabetic, post-trauma, arterial, venous) | Ulcer chronicity in the interventional group: 10.19 (SD: 4.37) months and 14.53 (9.75) months in the control group | Exp: ePRP:SVF (mechanical disruption) + PRP (plt: 4–7x) CTR: Standard wound care | Wound closure rate/18 month Lost to follow-up: 0 | Exp: 0.2287 cm/day vs. CTR: 0.0890 cm/day (p = 0.0257) No matched groups, baseline differences in ulcer area (EXP vs. CTR 29.59 cm2 vs. 8.5 cm2) |
| Walter (2011) [22] | RCT Exp N = 19 CTR N = 21 | Aterosclerotic patients | Nonhealing ulcers (Rutherford class 5 or 6) | Exp: autologous bone marrow-derived mononuclear cells (BM-MNC) CTR: Placebo | Complete healing/amputation-free survival/freedom from rest pain Lost to follow-up: 12 | Ulcer area decreased significantly in the BM-MNC (p < 0.014) but not in CTR group. Patients in CTR group switched to BM-MNC treatment and ulcer area decreased at 3 months. Repeated BM-MNC administration significantly correlated with complete ulcer healing |
| Author (Year) [Reference] | Study Design Experimental/Control Group | Patient Population | Chronicity of the Ulcer | Biological Intervention and Control Management | Outcomes/Follow-Up | Differences and Statistical Results |
|---|---|---|---|---|---|---|
| Ahmed (2017) [23] | RCT Exp N = 28 patients CTR N = 28 patients Matched wounds between groups | DFU 56 patients | >6 weeks | Exp: Autologous gelified PRP (4–5x) twice weekly CTR: antiseptic oilment | Ulcer healing, healing rate/8 weeks | Exp: 86% CTR: 68% Healing rate: Exp: 0.7 cm2/week CTR: 0.5 cm2/week |
| Anitua (2008) [24] | RCT (pilot) Exp N = 8 patients CTR N = 7 patients | 64% venous, 29% pressure, 7% other Baseline characteristics were not similar between groups | >4 weeks | Exp: Autologous gelified PRP (1.5–2.5x) CTR: Conventional treatment | Mean percentage of surface healed/8 weeks Lost to follow-up: 6 | Exp: 5 patients CTR: 4 Exp: 72.94% (SD: 22.25%) CTR: 21.48% (SD: 33.56%) p < 0.05 |
| Danielsen (2008) [25] | RCT Exp N = 10 patients CTR N = 10 patients | Graft surgery in patients with chronic leg ulcers (evaluation of meshed autografts and acute split thickness donor wounds) | Non-reported | Exp: platelet rich fibrin (Vivostat) CTR: saline Platelet rich fibrin sprayed into the donor and recipient wound plus three dressings (two different dressings and one polyurethane closure) | Wound Epithelialization Immunohistomorphometry pain/20 weeks | Epithelial coverage of donor wounds did not differ significantly between platelet-rich fibrin and control on day 5 or day 8 |
| Driver (2006) [26] | RCT Exp N = 19 patients CTR N = 21 patients | 72 patients with type I or II diabetes Efficacy analysis dropouts | >4 weeks | Exp: Platelet gel (autologel®) versus CTR: Placebo gel | Proportion of healed ulcers and time to healing 24 weeks | Exp: 13/16 CTR: 8/19 Time to healing shorter in Exp group (p = 0.018) 12 week treatment phase Safety evaluation |
| Jeong (2010) [27] | RCT Exp N = 52 patients CTR N = 48 patients | DFU | >4 weeks | Exp: Blood Bank Platelet Concentrate versus CTR: treatment with topical fibrinogen and thrombin | Complete wound healing was achieved/12 weeks | Exp: 79% CTR: 46% (p < 0.05) |
| Kakagia (2007) [28] | RCT Exp A N = 17 patients Exp B N = 17 patients Exp C N = 17 patients | 51 patients with significant tissue defects of the foot | >3 months | Exp A: oxidized cellulose/collagen Exp B: autologous PRP Exp C: a combination of both | Ulcer dimension within 8 week follow-up | No differences between groups |
| Karimi (2015) [29] | RCT Exp N = 25 patients CTR N = 25 patients | DFU | No limit | Exp: PRP CTRL: conventional management | Ulcer’s depth in three weeks | Exp: 4.56 ± 5.76 CTRL: 13.03 ± 14.1 p = 0.004 |
| Knighton (1990) [30] | RCT Exp N = 16 patients CTR N = 16 patients | 10 venous diseases, 9 diabetic, 4 occlusive peripheral vascular diseases, and 1 vasculitis | Differences in ulcer chronicity experimental group: 119 days (SD: 114) and control group: 47 days (SD: 63) | Exp: Autologous PDWHF + microcrystalline collagen (Avitene®) CTR: placebo (buffer solution + mycrocrystalline collagen) No plasma, platelets resuspended in buffer Topical application | Time to 100% of epithelialization/16 weeks Number of patients analyzed: 13 in PRP group and 11 in control group Lost to follow-up: 2 | Exp = 81% vs. CTR = 15% epithelialization p < 0.0001 |
| Krupski (1991) [31] | RCT Exp N = 10 patients CTR N = 8 patients | Number ulcers: 26 Wound aetiology: Mixed 78% diabetic, 72% occlusive peripheral vascular disease, and 28% venous disease | >8 weeks | Exp: PDWHF topical solution) every 12 h CTR: saline solution every 12 h The treatment is applied by the patient | Total epithelialization/12 weeks | Exp: 4/17 CTR: 3/9 Healing rate Exp: −4.3(12.2) cm2/week CTR: 1.9 (2.7) cm2/week |
| Li L (2015) [32] | RCT Exp N = 59 patients CTR N = 58 patients | DFU refractory | >2 weeks | Autologous platelet-rich gel, double spinning and calcium gluconate activation Repeated PR-gel application if reduction of wound area did not reach 80% reduction 2 weeks after treatment | Reduction rate at the end of week 12th/12 weeks Lost to follow-up Exp: 6, CTR: 5 | Healing velocity faster in PR-gel group, p = 0.020 |
| Moneib (2017) [33] | CT Exp N = 20 CTR N = 20 | Venous leg ulcer Ankle/brachial index > 0.80 | >6 months | PR-gel double spinning activation with calcium gluconate + compression CTR: saline management + compression | Reduction in ulcer size expressed as percentage improvement in area | Higher reduction in ulcer size in PRP group compared with control p < 0.0001 |
| Obolensky (2017) [34] | CT Exp N = 50 patients CTR N = 50 patients | Non-healing wounds of different etiology, 82% of the wounds located in lower limb | >6 weeks | Exp: Pure PRP (single spinning) CTR: conventional management | Epithelialization time Hospitalization time Economic effect | Epithelialization: Exp: 42.3 days (SD: 5.7) CTR: 123.8 days (SD: 25.3) Hospitalization Exp 8.4 days (SD: 1.5) CTR: 18.1 (SD: 1.6) €736.81 in savings per patient PRP group |
| Pravin (2016) [35] | RCT Exp1 N = 16 Exp2 N = 15 | 22 venous ulcers, 1 vasculitis, 1 traumatic, 2 diabetic, 4 trophic ulcers | >8 weeks | Exp1: PRP (double spinning) Exp2: L-PRF (single spinning) Weekly administration for 6 weeks | Study period 6 weeks, follow-up 6 weeks | Mean duration of healing: 5.7 weeks in L-PRF and 6.5 weeks in PRP p = 0.034 |
| Saad Setta (2011) [36] | RCT Exp N = 12 patients CTR N = 12 patients | Non healing DFUs | >3 months | Exp: gelified platelet-rich plasma (with bovine thrombin and CaCl2) CTR: platelet-poor plasma Treatment applied twice weekly until closure (maximum 20 weeks) | Healing duration in weeks/20 weeks Lost to follow-up: 3 | Exp: 11.5 weeks CTR: 17.1 weeks, p < 0.005 |
| Saldalamachia (2004) [37] | CT Exp N = 7 patients CTR N = 7 patients | Diabetic foot 15 patients | >8 weeks | Exp: autologous gelified PRP, topical application CTR: standard care Weekly application for 5 weeks No description of PRP product | Reduction rate = [(final area (mm2)—nitial area (mm2)/initial area (mm2)]/5 weeks Lost to follow-up: 1 | Reduction rate 71.9 (22.5) vs. 9.2 (67.8) p < 0.039 or reduction of 50% or more was Exp: 71% and CTR: 29% |
| Sennet (2003) [38] | RCT Exp N = 8 patients CTR N = 7 patients | Chronic venous leg ulcer | >2 months | Exp: frozen platelet lysate obtained by sonication 107 plt/cm2 in saline CTR: saline | Mean percent reduction in ulcer area/12 weeks | Exp: 26.2% CTR: 15.2% (p = 0.94). |
| Stacey (2000) [39] | RCT Exp N = 42 patients CTR N = 44 patients | Venous ulceration, with no other possible cause for poor healing | >3 months | Exp: autologous platelet lysate (without plasma) 2 × 109 plt/mL CTR: PBS | Ulcer healing/9 months Lost to follow-up: 11 | No significant differences between treatment Only ulcer size influenced healing |
| Steed (1992) [40] | RCT Exp N = 7 patients CTR N = 6 patients | 13 subjects with neurotrophic ulcer | >8 weeks | Exp: PDWHF (obtained from washed allogeneic platelets (without plasma) stimulated with thrombin CTR: placebo | Ulcer healing/Followed for 20 weeks | Exp: 5/7 ulcers healed by week 15th CTR: 1/6 ulcers healed by week 20th |
| Sources of Bias | Dash (2009) [13] | Dubsky (2013) [14] | Han (2009) [15] | Jain (2011) [16] | Kirana (2012) [17] | Lu (2011) [18] | Marino (2013) [19] | Procházka (2010) [20] | Raposio (2016) [21] | Walter (2011) [22] |
|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | ? | − | + | + | ? | + | − | ? | ? | ? |
| Allocation concealment (selection bias) | + | − | ? | + | ? | ? | − | ? | ? | ? |
| Blinding of patients (performance bias) | ? | − | − | ? | ? | + | − | ? | ? | ? |
| Blinding of personnel (performance bias) | ? | − | − | ? | ? | ? | − | ? | ? | ? |
| Incomplete outcome data (attrition bias) | + | + | + | + | + | + | ? | + | + | − |
| Selective reporting (reporting bias) | − | + | + | − | − | + | − | + | + | + |
| Other bias | + | + | ? | + | ? | + | ? | + | − | + |
| Sources of bias | Ahmed (2017) [23] | Anitua (2008) [24] | Danielsen (2008) [25] | Driver (2006) [26] | Jeong (2010) [27] | Kakagia (2007) [28] | Karimi (2015) [29] | Knighton (1990) [30] | Krupski (1991) [31] | Li (2015) [32] | Moneib (2017) [33] | Obolenski (2017) [34] | Pravin (2016) [35] | Saad Setta (2011) [36] | Saldalamacchia (2004) [37] | Senet (2003) [38] | Stacey (2000) [39] | Steed (1992) [40] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | ? | + | + | + | + | + | ? | ? | + | + | − | ? | ? | − | − | ? | + | ? |
| Allocation concealment (selection bias) | ? | ? | + | + | ? | ? | ? | + | + | + | − | ? | ? | − | − | ? | + | ? |
| Blinding of patients (performance bias) | ? | − | ? | + | ? | ? | ? | + | + | ? | − | ? | − | − | − | ? | ? | ? |
| Blinding of personnel (performance bias) | ? | − | ? | − | ? | ? | ? | − | + | ? | − | ? | − | − | − | ? | ? | ? |
| Incomplete outcome data (attrition bias) | ? | − | + | − | ? | + | + | − | + | + | + | + | ? | + | + | + | + | + |
| Selective reporting (reporting bias) | + | + | ? | + | + | − | + | − | + | + | − | ? | − | + | − | − | + | − |
| Other bias | ? | − | + | ? | ? | ? | + | − | + | + | ? | ? | ? | + | ? | + | + | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos-Alonso, N.; Lobato, I.; Hernández, I.; Sebastian, K.S.; Rodríguez, B.; Grandes, G.; Andia, I. Adjuvant Biological Therapies in Chronic Leg Ulcers. Int. J. Mol. Sci. 2017, 18, 2561. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122561
Burgos-Alonso N, Lobato I, Hernández I, Sebastian KS, Rodríguez B, Grandes G, Andia I. Adjuvant Biological Therapies in Chronic Leg Ulcers. International Journal of Molecular Sciences. 2017; 18(12):2561. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122561
Chicago/Turabian StyleBurgos-Alonso, Natalia, Igone Lobato, Igone Hernández, Kepa San Sebastian, Begoña Rodríguez, Gontzal Grandes, and Isabel Andia. 2017. "Adjuvant Biological Therapies in Chronic Leg Ulcers" International Journal of Molecular Sciences 18, no. 12: 2561. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122561