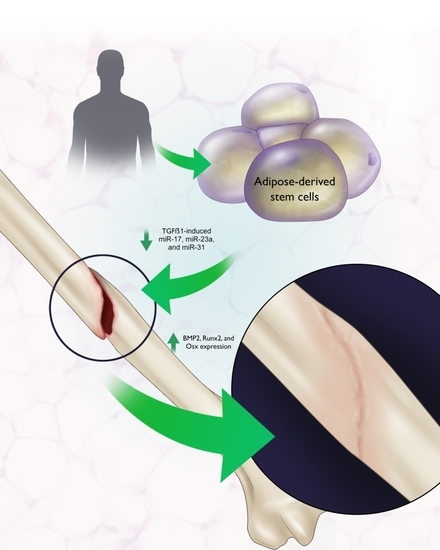
Function of microRNAs in the Osteogenic Differentiation and Therapeutic Application of Adipose-Derived Stem Cells (ASCs)
Abstract
:1. Introduction
2. Utilization of Adipose-Derived Stem Cells for Bone Repair
2.1. Tissue Sites for Harvesting ASCs
2.2. Donor Characteristics
2.3. Harvesting and Isolating Adipose-Derived Stem Cells
2.4. Surface Markers for Validating Cell Populations
3. MicroRNAs mediating ASC osteogenic differentiation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W. Adipose-Derived Stem Cells: Implications in Tissue Regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Zhu, M.; Wulur, I.; Alfonso, Z. Adipose-Derived Stem Cells. Methods Mol. Biol. 2008, 449, 59–67. [Google Scholar] [PubMed]
- Gelberman, R.H.; Shen, H.; Kormpaki, I.; Rothrauff, B.; Yang, G.; Tuan, R.S.; Xia, Y.; Sakiyama-Elbert, S.; Silva, M.J.; Thomopoulos, S. Effect of adipose-derived stromal cells and BMP12 on intrasynovial tendon repair: A biomechanical, biochemical, and proteomics study. J. Orthop. Res. 2016, 34, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Tapp, H.; Hanley, E.N., Jr.; Patt, J.C.; Gruber, H.E. Adipose-derived stem cells: Characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. (Maywood) 2009, 234, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Lee, S.Y.; Sakai, Y.; Nishida, K.; Kuroda, R.; Kurosaka, M. Causative factors of fracture nonunion: The proportions of mechanical, biological, patient-dependent, and patient-independent factors. J. Orthop. Sci. 2014, 9, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Albisetti, W.; Agus, A.; Lori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 2007, 38, S11–S18. [Google Scholar] [CrossRef]
- Fulkerson, E.; Egol, K.A.; Kubiak, E.N.; Liporace, F.; Kummer, F.J.; Koval, K.J. Fixation of diaphyseal fractures with a segmental defect: A biomechanical comparison of locked and conventional plating techniques. J. Trauma 2006, 60, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Majumder, S.; Batchelor, A.G.; Knight, S.L.; De Boer, P.; Smith, R.M. Fix and flap: The radical orthopaedic and plastic treatment of severe open fractures of the tibia. J. Bone Jt. Surg. Br. 2000, 82, 959–966. [Google Scholar] [CrossRef]
- Cattaneo, R.; Catagni, M.; Johnson, E.E. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin. Orthop. Relat. Res. 1992, 280, 143–152. [Google Scholar] [CrossRef]
- Fernyhough, J.C.; Schimandle, J.J.; Weigel, M.C.; Edwards, C.C.; Levine, A.M. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine 1992, 17, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Sandor, G.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerström, B.; Patrikoski, M.; Seppänen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with crano-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Saxer, F.; Scherberich, A.; Todorov, A.; Studer, P.; Miot, S.; Schreiner, S.; Güven, S.; Tchang, L.A.; Haug, M.; Heberer, M.; et al. Implantation of Stromal Vascular Fraction Progenitors at Bone Fracture Sites: From a Rat Model to a First-in-Man Study. Stem Cells 2016, 34, 2956–2966. [Google Scholar] [CrossRef] [PubMed]
- Hicok, K.C.; Du Laney, T.V.; Zhou, Y.S.; Halvorsen, Y.D.C.; Hitt, D.C.; Cooper, L.F.; Gimble, J.M. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004, 10, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef] [PubMed]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Bucholz, R.W.; Bosse, M.J.; Mirza, S.K.; Lyon, T.R.; Webb, L.X.; Pollak, A.N.; Golden, J.D.; Valentin-Opran, A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J. Bone Jt. Surg. Am. 2006, 88, 1431–1441. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Leendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Z. Micrornas Modulate Hematopoietic Lineage Differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Karp, X. Developmental Biology Enhanced: Encountering MicroRnas in Cell Fate Signaling. Science 2005, 310, 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Poy, M.N.; Stoffel, M.; Fuchs, E. A Skin Microrna Promotes Differentiation by Repressing ‘Stemness’. Nature 2008, 7184, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Wang, S.; Wei, J.; Fan, J.; Li, J.; Han, Q.; Liao, L.; Shao, C.; Zhao, R.C. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013, 10, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Bian, C.; Li, J.; Du, Z.; Zhou, H.; Yang, Z.; Zhao, R.C. miR-21 modulates the ERK-MAPK singaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J. Cell. Biochem. 2013, 114, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of Microrna Expression Profiles in Normal Human Tissues. BMC Genom. 2007, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kong, X.; Liu, J.; Lv, Y.; Sheng, Y.; Lv, S.; Di, W.; Wang, C.; Zhang, F.; Ding, G. Expression Profiling of PPARγ-Regulated MicroRNAs in Human Subcutaneous and Visceral Adipogenesis in both Genders. Endocrinology 2014, 155, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.P.; Picard, D. Multidirectional Interplay between Nuclear Receptors and Micrornas. Curr. Opin. Pharmacol. 2010, 10, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Berthold, S.; Kovacs, P.; Schön, M.R.; Fasshauer, M.; Ruschke, K.; Stumvoll, M.; Blüher, M. Microrna Expression in Human Omental and Subcutaneous Adipose Tissue. PLoS ONE 2009, 4, e4699. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Gordon, J.A.; Beloti, M.M.; Croce, C.M.; van Winjnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. A network connecting Runx2, STATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation progam. Proc. Natl. Acad. Sci. USA 2010, 107, 19879–19884. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chang, Y.; Sung, L.; Li, K.; Yeh, C.; Yen, T.; Hwang, S.; Lin, K.; Hu, Y. Osteogenic differentiation of adipose-derived stem cells and calvarial defect repair using baculovirus-mediated co-expression of BMP-2 and miR-148b. Biomaterials 2014, 35, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hassan, M.Q.; Jafferji, M.; Aqeilan, R.I.; Garzon, R.; Croce, C.M.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2009, 284, 15676–15684. [Google Scholar] [CrossRef] [PubMed]
- Peptan, I.A.; Hong, L.; Mao, J.J. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast. Reconstr. Surg. 2006, 117, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Thomou, T.; DePonte, M.; Koo, A.; Forse, R.A.; Chinnappan, D.; Martin-Ruiz, C.; Von Zglinicki, T.; et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 2006, 55, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Aksu, A.E.; Rubin, J.P.; Dudas, J.R.; Marra, K.G. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann. Plast. Surg. 2008, 60, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Van Harmelen, V.; Skurk, T.; Rohrig, K.; Lee, Y.W.; Halbleib, M.; Aprath-Husmann, I.; Hauner, H. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Beane, S.; Fonseca, V.C.; Cooper, L.; Koren, G.; Darling, E. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Tamski, H.; Cook, C.; Sandtanam, N. MicroRNA regulation of adipose derived stem cells in aging rats. PLoS ONE 2013, 8, e59238. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Rodbell, M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringes alpha toxin) and of insulin on glucose and amino acid metabolism. J. Biol. Chem. 1966, 241, 130–139. [Google Scholar] [PubMed]
- Zhu, M.; Heydarkhan-Hagvall, S.; Hedrick, M.; Benhaim, P.; Zuk, P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J. Vis. Exp. 2013, 79, e50585. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Scherberich, A.; Di Maggio, N.D.; McNagny, K.M. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J. Stem Cells 2013, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.J.; Tholpady, A.; Tholpady, S.S.; Shang, H.; Ogle, R.C. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 2005, 23, 412–442. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Derzins, U.; Nikulshin, S.; Skrastina, D.; Ezerta, A.; Legzdina, D.; Kozlovska, T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J. Stem Cells 2014, 9, 135–148. [Google Scholar] [PubMed]
- Li, H.; Zimmerlin, L.; Marra, K.G.; Donnenberg, V.S.; Donnenberg, A.D.; Rubin, J.P. Adipogenic potential of adipose stem cell subpopulations. Plast. Reconstr. Surg. 2011, 128, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Lin, G.; Xin, Z.; Zhang, H.; Banie, L.; Wang, G.; Qiu, X.; Ning, H.; Luem, T.F.; Lin, C.S. Identification of active and quiescent adipose vascular stromal cells. Cytotherapy 2012, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericytes and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, Y.D.; Franklin, D.; Bond, A.L.; Hitt, D.C.; Auchter, C.; Boskey, A.L.; Paschalis, E.P.; Wilkison, W.O.; Gimble, J.M. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001, 7, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fu, R.; Shyu, W.; Liu, S.; Jong, G.; Chiu, Y.; Wu, H.; Tsou, Y.; Cheng, C.; Lin, S. Adipose-derived stem cells: Isolation, characterization, and differentiation potential. Cell Transp. 2013, 22, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Chiellini, C.; Massoudi, A.; Cochet, O.; Zaragosi, L.E.; Trojani, C.; Michiels, J.F.; Weiss, P.; Carle, G.; Rochet, N.; et al. Human adipose tissue-derived multipotent stem cells differentiate in vitro and in vivo into osteocyte-like cells. Biochem. Biophys. Res. Commun. 2007, 361, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, R.L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 8, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Luzi, E.; Marini, F.; Sala, S.C.; Tognarini, I.; Galli, G.; Brandi, M.L. Osteogenic Differentiation of human adipose tissue-derived stem cells is moduled by the miR26a targeting of SMAD1 transcription factor. J. Bone Miner. Res. 2008, 23, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Trompeter, H.I.; Dreesen, J.; Hermann, E.; Iwaniuk, K.M.; Hafner, M.; Renwick, N.; Tuschl, T.; Wernet, P. MicroRNAs miR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genom. 2013, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wei, W.; Ruan, J.; Ding, Y.; Zhuang, A.; Bi, X.; Sun, H.; Gu, P.; Wang, Z.; Fan, X. Effects of miR-146a on the osteogenesis of adipose-derived mesenchymal stem cells and bone regeneration. Sci. Rep. 2017, 7, 42840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zhang, H.; Kang, Y.; Sheng, P.Y.; Ma, Y.C.; Yang, Z.B.; Zhang, Z.Q.; Fu, M.; He, A.S.; Liao, W.M. miRNA Expression Profile during Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J. Cell. Biochem. 2012, 113, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Baglìo, S.R.; Devescovi, V.; Granchi, D.; Baldini, N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene 2013, 527, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, Y.; Zhang, S.; Jia, L.; Zhou, Y. Promotion effects of mir-375 on the osteogenic differentiation of human adipose-derived mesenchymal stem cells. Stem Cell Rep. 2017, 8, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, Q.; Yu, Z.; Zhou, H.; Huang, Y.; Bi, X.; Wang, Y.; Shi, W.; Sun, H.; Gu, P.; et al. A regulatory loop containing miR-26a, GSK3β and C/EBPα regulates the osteogenesis of human adipose-derived mesenchymal stem cells. Sci. Rep. 2015, 5, 15280. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.C.; Qureshi, A.; Dasa, V.; Freitas, M.; Gimble, J.M.; Davis, T.A. MicroRNA regulation of stem cell differentiation and siseases of bone and adipose tissue: Perspectives on mimiRNA biogenesis and cellular transcriptome. Biochimie 2016, 124, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, G.S.; Styer, A.M.; Strodel, W.E.; Roesch, S.L.; Yavorek, A.; Carey, D.J.; Wood, G.C.; Petrick, A.T.; Gabrielsen, J.; Ibele, A.; et al. Gene expression profiling in subcutaneous, visceral, and epigastric adipose tissues of patients with extreme obesity. Int. J. Obes. 2014, 38, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Miselli, M.A.; Sanz, J.M.; Dalla Nora, E.; Morieri, M.L.; Colonna, R.; Pišot, R.; Zuliani, G. Gene expression regional differences in human subcutaneous adipose tissue. BMC Genom. 2017, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.K.; Ashraf, M. Preconditioning and stem cell survival. J. Cardiovasc. Transl. Res. 2010, 3, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Katsura, A.; Suzuki, H.I.; Ueno, T.; Mihira, H.; Yamazaki, T.; Yasuda, T.; Watabe, T.; Mano, H.; Yamada, Y.; Miyazono, K. MicroRNA-31 is a positive modulator of endothelial-mesenchymal transition and associated secretory phenotype induced by TGF-β. Genes Cells 2016, 21, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, C.; Liu, Q.; Liu, B.; Song, C.; Zhu, S.; Wu, C.; Liu, S.; Yu, H.; Yao, D.; et al. The role of the miR-31/FIH1 pathway in TGF-β-induced liver fibrosis. Clin. Sci. (Lond.) 2015, 129, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Wang, A.; Meisgen, F.; Pivarcsi, A.; Sonkoly, E.; Ståhle, M.; Landén, N.X. MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. Investig. Dermatol. 2015, 135, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Butz, H.; Rácz, K.; Hunyady, L.; Patócs, A. Crosstalk between TGF-β signaling and the microRNA machinery. Trends Pharmacol. Sci. 2012, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Wang, Y.; Liang, J.; Qiu, T.; Wang, H.; Zhang, Y.; Zhang, Y.; Hui, Q.; Tao, K. Screening for genes, transcription factors and miRNAs associated with the myogenic and osteogenic differentiation of human adipose tissue-derived stem cells. Int. J. Mol. Med. 2016, 38, 1839–1849. [Google Scholar] [CrossRef] [PubMed]



| Stromal Vascular Fraction |
| Positive: CD11, CD13, CD14 (+/−), CD29, CD31, CD34(+/−), CD44, CD45 (+/−), CD49d, CD49e, CD55, CD63, CD73, CD90, CD105 (+/−), CD106 (+/−), CD117, CD144, CD146, CD166 (+/−), HLA-DR |
| Negative: CD11b, CD19, CD56, STRO-1 |
| Adipose-Derived Stem Cells |
| Positive: CD9, CD10, CD13, CD29, CD44, CD49d, CD49e, CD54, CD55, CD63, CD73, CD90, CD105, CD144, CD146, CD166, HLA-ABC, CD34 (+/−), STRO-1 |
| Negative: CD3, CD11b, CD14, CD19, CD31, CD34, CD45, CD56, CD62L, CD96L, CD117, HLA-DR |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodges, W.M.; O’Brien, F., III; Fulzele, S.; Hamrick, M.W. Function of microRNAs in the Osteogenic Differentiation and Therapeutic Application of Adipose-Derived Stem Cells (ASCs). Int. J. Mol. Sci. 2017, 18, 2597. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122597
Hodges WM, O’Brien F III, Fulzele S, Hamrick MW. Function of microRNAs in the Osteogenic Differentiation and Therapeutic Application of Adipose-Derived Stem Cells (ASCs). International Journal of Molecular Sciences. 2017; 18(12):2597. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122597
Chicago/Turabian StyleHodges, Walter M., Frederick O’Brien, III, Sadanand Fulzele, and Mark W. Hamrick. 2017. "Function of microRNAs in the Osteogenic Differentiation and Therapeutic Application of Adipose-Derived Stem Cells (ASCs)" International Journal of Molecular Sciences 18, no. 12: 2597. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122597