In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite
Abstract
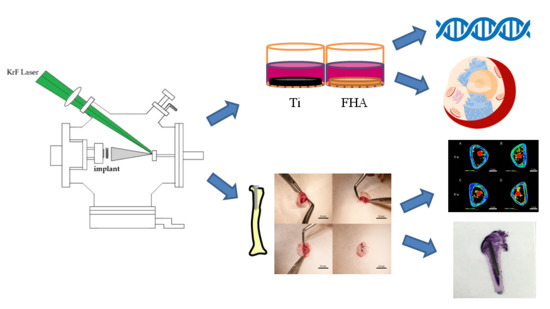
:1. Introduction
2. Results
2.1. Materials Fabrication
2.2. Surface Characterization
2.3. Real-Time Quantitative Polymerase Chain Reaction (PCR)
2.4. Calcium Deposition in the Extracellular Matrix and Osteocalcin Production
2.5. Implantation into Rat Femurs
2.6. Microcomputed Tomography
2.7. Histology and Sequential Fluorescent Labeling
3. Discussion
4. Materials and Methods
4.1. Materials Fabrication
4.2. Surface Characterization
4.3. Cell Culture
4.4. Real-Time Quantitative PCR
4.5. Calcium Deposition in the Extracellular Matrix and Osteocalcin Production
4.6. Implantation into Rat Femurs
4.7. Sequential Fluorescent Labeling and Microcomputed Tomography
4.8. Histology of Sequentially Labeled Sections
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| KrF | krypton fluoride |
| FHA | fluoridated hydroxyapatite |
| Ti | titanium |
| PLD | pulsed laser deposition |
| SEM | scanning electron microscopy |
| SPM | scanning probe microscopy |
| ATR-FTIR | attenuated reflectance Fourier transform infrared spectroscopy |
| PCR | polymerase chain reaction |
| ALP | alkaline phosphatase |
| RUNX2 | runt-related transcription factor 2 |
| BMP | bone morphogenetic protein |
| BV/TV | bone volume to total volume |
| Tb.N | mean trabecular number |
| Tb.Th | mean trabecular thickness |
| Tb.Sp | mean trabecular separation |
| BA | bone area ratio |
| BIC | bone–implant contact |
| LBA | labeled bone area |
References
- Amrollahi, P.; Shah, B.; Seifi, A.; Tayebi, L. Recent advancements in regenerative dentistry: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, K. Oral rehabilitation considerations for partially edentulous periodontal patients. J. Prosthodont. 2012, 21, 494–513. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.S.; Kern, T.; Wolfart, S.; Heussen, N. A systematic review and meta-analysis of removable and fixed implant-supported prostheses in edentulous jaws: Post-loading implant loss. Clin. Oral Implants Res. 2016, 27, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, P.; van der Linden, W.; van Eeden, R. Optimal restoration of dental esthetics and function with advanced implant-supported prostheses: A clinical report. J. Prosthodont. 2012, 21, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Schwartz Filho, H.O.; Wennerberg, A. The influence of surface treatment on the implant roughness pattern. J. Appl. Oral Sci. 2012, 20, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Jaffin, R.A.; Berman, C.L. The excessive loss of Branemark fixtures in type IV bone: A 5-year analysis. J. Periodontol. 1991, 62, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.; Harun, W.S.; Hassan, M.A.; Ghani, S.A.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Kim, D.H.; Kovacik, P.; Sojoudi, H.; Wang, M.; Gleason, K.K. Polymer thin films and surface modification by chemical vapor deposition: Recent progress. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, G.; Umar, A. Pulse laser deposited nanostructured ZnO thin films: A review. J. Nanosci. Nanotechnol. 2014, 14, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C. Efficient CH3 NH3 PbI3 perovskite solar cells employing nanostructured p-Type NiO electrode formed by a pulsed laser deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Hontsu, S.; Nakamori, M.; Tabata, H.; Ishii, J.; Kawai, T. Pulsed laser deposition of bioceramic hydroxyapatite thin films on polymer materials. Jpn. J. Appl. Phys. 1996, 35, L1208. [Google Scholar]
- Matsumoto, T.; Kanno, T.; Hontsu, S.; Hosoi, Y.; Kato, N.; Demizu, K.; Uenoya, T.; Sugimura, T. Formation of biocompatible hydroxyapatite thin films deposited by laser ablation. Bioceramics 1999, 12, 499–502. [Google Scholar]
- Schöning, M.J.; Mourzina, Y.G.; Schubert, J.; Zander, W.; Legin, A.; Vlasov, Y.G.; Lüth, H. Pulsed laser deposition—An innovative technique for preparing inorganic thin films. Electroanalysis 2010, 13, 727–732. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.M.; Clèries, L.; Sardin, G.; Morenza, J.L. Characterization of calcium phosphate coatings deposited by Nd:YAG laser ablation at 355 nm: Influence of thickness. Biomaterials 2002, 23, 1989–1994. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, J.H.; Koh, J.T.; Lim, H.P.; Oh, G.J.; Lee, S.W.; Lee, K.M.; Yun, K.D.; Park, S.W. Preparation and characterisation of hydroxyapatite coatings on nanotubular TiO2 surface obtained by sol-gel process. J. Nanosci. Nanotechnol. 2015, 15, 6164–6167. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hao, L.; Hussein, A.; Wei, Q.; Shi, Y. Microstructural and surface modifications and hydroxyapatite coating of Ti-6Al-4V triply periodic minimal surface lattices fabricated by selective laser melting. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Tsai, T.B.; Say, W.C.; Chiu, C.; Chen, S.H. In vitro study of electrodeposited fluoridated hydroxyapatite coating on G-II titanium with a nanostructured TiO2 interlayer. Biomed. Mater. 2017, 12, 025018. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S. The mineral of bone. Clin. Orthop. Relat. Res. 1985, 200, 87–99. [Google Scholar] [CrossRef]
- Kim, H.W.; Noh, Y.J.; Koh, Y.H.; Kim, H.E. Enhanced performance of fluorine substituted hydroxyapatite composites for hard tissue engineering. J. Mater. Sci. Mater. Med. 2003, 14, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chao, Y.; Wan, Q.; Zhu, Z.; Yu, H. Fluoridated hydroxyapatite coatings on titanium obtained by electrochemical deposition. Acta Biomater. 2009, 5, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Barinov, S.M.; Tumanov, S.V.; Fadeeva, I.V.; Bibikov, V.Y. Environment effect on the strength of hydroxy- and fluorohydroxyapatite ceramics. Inorg. Mater. 2003, 39, 877–880. [Google Scholar] [CrossRef]
- Overgaard, S.; Lind, M.; Glerup, H.; Grundvig, S.; Bünger, C.; Søballe, K. Hydroxyapatite and fluorapatite coatings for fixation of weight loaded implants. Clin. Orthop. Relat. Res. 1997, 336, 286–296. [Google Scholar] [CrossRef]
- Li, Z.; Huang, B.; Mai, S.; Wu, X.; Zhang, H.; Qiao, W.; Luo, X.; Chen, Z. Effects of fluoridation of porcine hydroxyapatite on osteoblastic activity of human MG63 cells. Sci. Technol. Adv. Mater. 2015, 16, 035006. [Google Scholar] [CrossRef] [PubMed]
- Alradha, A.S.D. Impact of anxiety on the satisfaction of dental implant patients. J. Prosthodont. 2017. [Google Scholar] [CrossRef] [PubMed]
- Assadian, M.; Jafari, H.; Ghaffari Shahri, S.M.; Idris, M.H.; Almasi, D. Topography, wetting, and corrosion responses of electrodeposited hydroxyapatite and fluoridated hydroxyapatite on magnesium. Biomed. Mater. Eng. 2016, 27, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzo, L.M.; Hart, J.N.; Gross, K.A. Influence of fluorine in the synthesis of apatites. Synthesis of solid solutions of hydroxy-fluorapatite. Biomaterials 2003, 24, 3777–3785. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Bemporad, E.; Sabastiani, M. F-substituted hydroxyapatite nanopowders: Thermal stability, sintering behaviour and mechanical properties. Ceram. Int. 2010, 36, 313–322. [Google Scholar] [CrossRef]
- Jelínek, M.; Vaněk, P.; Tolde, Z.; Buixaderas, E.; Kocourek, T.; Studnička, V.; Drahokoupil, J.; Petzelt, J.; Remsa, J.; Tyunina, M. PLD prepared bioactive BaTiO3 films on TiNb implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Kubokawa, O.; Nanbu, K.; Fujimoto, K.; Matsumoto, Y. Combinatorial synthesis of epitaxial LiCoO2 thin films on SrTiO3(001) via on-substrate sintering of Li2CO3 and CoO by pulsed laser deposition. ACS Comb. Sci. 2016, 18, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology—from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.E.; Meyer, A.E.; Natiella, J.R.; Natiella, R.R.; Carter, J.M. Surface properties determine bioadhesive outcomes: Methods and results. J. Biomed. Mater. Res. 1984, 18, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Štefková, K.; Procházková, J.; Pacherník, J. Alkaline phosphatase in stem cells. Stem Cells Int. 2015, 2015, 628368. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, R.; Komasa, S.; Xue, Y.; Jin, B.; Hou, Y.; Okazaki, J.; Gao, J. Drug-loadable calcium alginate hydrogel system for use in oral bone tissue repair. Int. J. Mol. Sci. 2017, 18, 989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Legeros, R.Z.; Silverstone, L.M.; Daculsi, G.; Kerebel, L.M. In vitro caries-like lesion formation in F-containing tooth enamel. J. Dent. Res. 1983, 62, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Weng, W.; Cheng, K.; Du, P.; Shen, G.; Han, G.; Huang, Y.; Yan, W.; Zhang, S. In vitro bioactivity and osteoblast-like cell test of zinc containing fluoridated hydroxyapatite films. J. Mater. Sci. Mater. Med. 2007, 18, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Wei, M. The effect of fluoride contents in fluoridated hydroxyapatite on osteoblast behavior. Acta Biomater. 2006, 2, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Karlis, G.A.; Anderson, G.I.; Dunstan, C.R.; Carbone, A.; Berger, G.; Ploska, U.; Zreiqat, H. Bone growth is enhanced by novel bioceramic coatings on Ti alloy implants. J. Biomed. Mater. Res. A 2009, 90, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Pires, J.T.; Shibli, J.A.; Mijiritsky, E.; Iezzi, G.; Piattelli, A.; Mangano, C. Early bone response to dual acid-etched and machined dental implants placed in the posterior maxilla: A histologic and histomorphometric human study. Implant Dent. 2017, 26, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.M.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Mizuno, K.; Kurokawa, K.; Ohkuma, S. Regulatory mechanisms and pathophysiological significance of IP3 receptors and ryanodine receptors in drug dependence. J. Pharmacol. Sci. 2013, 123, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Galione, A. Two-pore channels: Current controversies. Bioessays 2014, 36, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, Y.; Gao, Q.; Niu, Q.; Shen, M.; Fu, Q.; Hu, K.; Kong, L. Effects of a micro/nano rough strontium-loaded surface on osseointegration. Int. J. Nanomed. 2015, 10, 4549–4563. [Google Scholar]
- Abron, A.; Hopfensperger, M.; Thompson, J.; Cooper, L.F. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J. Prosthet. Dent. 2001, 85, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Ueda, M.; Kohiga, Y.; Imura, K.; Hontsu, S. Application of fluoridated hydroxyapatite thin film coatings using KrF pulsed laser deposition. Dent. Mater. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR induced by alkali treatment. Int. J. Mol. Sci. 2017, 18, 780. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surfaces with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.Y.; Lee, S.W.; Hahn, B.D.; Lee, Y.C.; Kim, S.G. Hydroxyapatite and silk combination-coated dental implants result in superior bone formation in the peri-implant area compared with hydroxyapatite and collagen combination-coated implants. J. Oral Maxillofac. Surg. 2014, 72, 1928–1936. [Google Scholar] [CrossRef] [PubMed]













| Device | Ra (nm) |
|---|---|
| Ti | 5.83 ± 0.98 |
| FHA | 24.48 ± 2.94 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite. Int. J. Mol. Sci. 2018, 19, 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19041127
Chen L, Komasa S, Hashimoto Y, Hontsu S, Okazaki J. In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite. International Journal of Molecular Sciences. 2018; 19(4):1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19041127
Chicago/Turabian StyleChen, Luyuan, Satoshi Komasa, Yoshiya Hashimoto, Shigeki Hontsu, and Joji Okazaki. 2018. "In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite" International Journal of Molecular Sciences 19, no. 4: 1127. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19041127