Central Nervous System Responses to Simulated Galactic Cosmic Rays
Abstract
:1. Introduction
2. The Effects of HZE Particle Irradiation on Behavioral and Cognitive Functions in Rodents
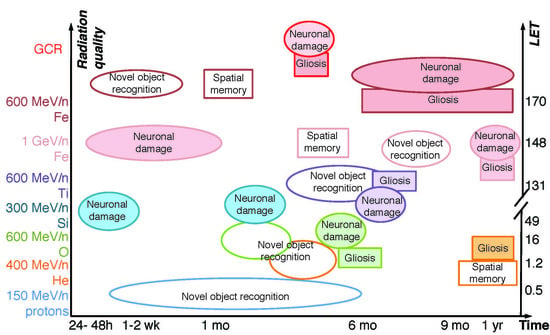
3. Individual HZE Ion and Combined GCR Effects on Neuronal Damage and Neuroinflammation in Rodents
4. Experimental and Epidemiological Variables That Influence HZE Ion Effects in the CNS
5. Novel Approaches for Investigating CNS Responses of Humans to Simulated Cosmic Radiation and Countermeasure Development
6. Conclusions
Funding
Conflicts of Interest
References
- Nelson, G.A. Space Radiation and Human Exposures, A Primer. Radiat. Res. 2016, 185, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mewaldt, R.A. Galactic cosmic ray composition and energy spectra. Adv. Space Res. 1994, 14, 737–747. [Google Scholar] [CrossRef]
- Chatterjee, A.; Maccabee, H.D.; Tobias, C.A. Radial cutoff LET and radial cutoff dose calculations for heavy charged particles in water. Radiat. Res. 1973, 54, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Tobias, C.A.; Lyman, J.T.; Chatterjee, A.; Howard, J.; Maccabee, H.D.; Raju, M.R.; Smith, A.R.; Sperinde, J.M.; Welch, G.P. Radiological physics characteristics of the extracted heavy ion beams of the bevatron. Science 1971, 174, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Heuskin, A.C.; Osseiran, A.I.; Tang, J.; Costes, S.V. Simulating Space Radiation-Induced Breast Tumor Incidence Using Automata. Radiat. Res. 2016, 186, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krukowski, K.; Grue, K.; Frias, E.S.; Pietrykowski, J.; Jones, T.; Nelson, G.; Rosi, S. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav. Immun. 2018. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0889159118304173 (accessed on 11 August 2018). [CrossRef] [PubMed]
- Acharya, M.M.; Christie, L.A.; Lan, M.L.; Donovan, P.J.; Cotman, C.W.; Fike, J.R.; Limoli, C.L. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19150–19155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, V.K.; Allen, B.D.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.K.; Chmielewski, N.N.; Giedzinski, E.; Acharya, M.M.; Britten, R.A.; et al. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haley, G.E.; Yeiser, L.; Olsen, R.H.; Davis, M.J.; Johnson, L.A.; Raber, J. Early effects of whole-body 56Fe irradiation on hippocampal function in C57BL/6J mice. Radiat. Res. 2013, 179, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.; Jopson, T.; Pelz, C.; Tafessu, A.; Fareh, F.; Zuloaga, D.; Marzulla, T.; Riparip, L.K.; Stewart, B.; Rosi, S.; et al. Short-and long-term effects of 56Fe irradiation on cognition and hippocampal DNA methylation and gene expression. BMC Genomics 2016, 17, 825. [Google Scholar] [CrossRef] [PubMed]
- Tseng, B.P.; Giedzinski, E.; Izadi, A.; Suarez, T.; Lan, M.L.; Tran, K.K.; Acharya, M.M.; Nelson, G.A.; Raber, J.; Parihar, V.K.; et al. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid. Redox Signal 2014, 20, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.; Jopson, T.; Pelz, C.; Tafessu, A.; Fareh, F.; Zuloaga, D.; Marzulla, T.; Riparip, L.K.; Stewart, B.; Rosi, S.; et al. Bi-directional and shared epigenomic signatures following proton and 56Fe irradiation. Sci. Rep. 2017, 7, 10227. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Feng, X.; Paladini, M.S.; Chou, A.; Sacramento, K.; Grue, K.; Riparip, L.K.; Jones, T.; Campbell-Beachler, M.; Nelson, G.; et al. Temporary microglia-depletion after cosmic radiation modifies phagocytic activity and prevents cognitive deficits. Sci. Rep. 2018, 8, 7857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, V.K.; Allen, B.D.; Tran, K.K.; Chmielewski, N.N.; Craver, B.M.; Martirosian, V.; Morganti, J.M.; Rosi, S.; Vlkolinsky, R.; Acharya, M.M.; et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid. Redox Signal. 2015, 22, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Allen, A.R.; Sharma, S.; Allen, B.; Rosi, S.; Olsen, R.H.; Davis, M.J.; Eiwaz, M.; Fike, J.R.; Nelson, G.A. Effects of Proton and Combined Proton and 56Fe Radiation on the Hippocampus. Radiat. Res. 2016, 185, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Rosi, S.; Belarbi, K.; Ferguson, R.A.; Fishman, K.; Obenaus, A.; Raber, J.; Fike, J.R. Trauma-induced alterations in cognition and Arc expression are reduced by previous exposure to 56Fe irradiation. Hippocampus 2012, 22, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Ueno, M.; Anzai, K. Memory impairment, oxidative damage and apoptosis induced by space radiation: Ameliorative potential of alpha-lipoic acid. Behav. Brain Res. 2008, 187, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.A.; Davis, L.K.; Johnson, A.M.; Keeney, S.; Siegel, A.; Sanford, L.D.; Singletary, S.J.; Lonart, G. Low (20 cGy) doses of 1 GeV/u 56Fe-Particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat. Res. 2012, 177, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Liu, B.; Frost, J.L.; Lemere, C.A.; Williams, J.P.; Olschowka, J.A.; O’Banion, M.K. Galactic cosmic radiation leads to cognitive impairment and increased abeta plaque accumulation in a mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e53275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, V.K.; Maroso, M.; Syage, A.; Allen, B.D.; Angulo, M.C.; Soltesz, I.; Limoli, C.L. Persistent nature of alterations in cognition and neuronal circuit excitability after exposure to simulated cosmic radiation in mice. Exp. Neurol. 2018, 305, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Rudobeck, E.; Bellone, J.A.; Szucs, A.; Bonnick, K.; Mehrotra-Carter, S.; Badaut, J.; Nelson, G.A.; Hartman, R.E.; Vlkolinsky, R. Low-dose proton radiation effects in a transgenic mouse model of Alzheimer’s disease-implications for space travel. PLoS ONE 2017, 12, e0186168. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Jones, T.; Campbell-Beachler, M.; Nelson, G.; Rosi, S. Peripheral T Cells as a Biomarker for Oxygen-Ion-Radiation-Induced Social Impairments. Radiat. Res. 2018, 190, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mange, A.; Cao, Y.; Zhang, S.; Hienz, R.D.; Davis, C.M. Whole-Body Oxygen (16O) Ion-Exposure-Induced impairments in social odor recognition memory in rats are dose and time dependent. Radiat. Res. 2018, 189, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabin, B.M.; Joseph, J.A.; Shukitt-Hale, B. A longitudinal study of operant responding in rats irradiated when 2 months old. Radiat. Res. 2005, 164, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.M.; Joseph, J.A.; Shukitt-Hale, B. Effects of age and diet on the heavy particle-induced disruption of operant responding produced by a ground-based model for exposure to cosmic rays. Brain Res. 2005, 1036, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; DeCicco-Skinner, K.L.; Roma, P.G.; Hienz, R.D. Individual differences in attentional deficits and dopaminergic protein levels following exposure to proton radiation. Radiat. Res. 2014, 181, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; DeCicco-Skinner, K.L.; Hienz, R.D. Deficits in sustained attention and changes in dopaminergic protein levels following exposure to proton radiation are related to basal dopaminergic function. PLoS ONE 2015, 10, e0144556. [Google Scholar] [CrossRef] [PubMed]
- Sweet, T.B.; Hurley, S.D.; Wu, M.D.; Olschowka, J.A.; Williams, J.P.; O’Banion, M.K. Neurogenic Effects of Low-Dose Whole-Body HZE (Fe) Ion and Gamma Irradiation. Radiat. Res. 2016, 186, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whoolery, C.W.; Walker, A.K.; Richardson, D.R.; Lucero, M.J.; Reynolds, R.P.; Beddow, D.H.; Clark, K.L.; Shih, H.Y.; LeBlanc, J.A.; Cole, M.G.; et al. Whole-Body exposure to 28Si-Radiation Dose-Dependently disrupts dentate gyrus neurogenesis and proliferation in the short term and new neuron survival and contextual fear conditioning in the long term. Radiat. Res. 2017, 188, 532–551. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Rodriguez, O.C.; Winters, T.A.; Fornace, A.J., Jr.; Albanese, C.; Datta, K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging (Albany NY) 2013, 5, 607–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, P.; Obenaus, A.; Heffron, D.; Mandell, J. High-energy (HZE) radiation exposure causes delayed axonal degeneration and astrogliosis in the central nervous system of rats. Gravit. Space Res. 2007, 20, 89–91. [Google Scholar]
- Sokolova, I.V.; Schneider, C.J.; Bezaire, M.; Soltesz, I.; Vlkolinsky, R.; Nelson, G.A. Proton radiation alters intrinsic and synaptic properties of CA1 pyramidal neurons of the mouse hippocampus. Radiat. Res. 2015, 183, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Chowdhury, S.; Ma, R.; Le, K.X.; Hong, S.; Caldarone, B.J.; Stevens, B.; Lemere, C.A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 2017, 9, eaaf6295. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Colodner, K.J.; Matousek, S.B.; Merry, K.; Hong, S.; Kenison, J.E.; Frost, J.L.; Le, K.X.; Li, S.; Dodart, J.C.; et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci 2015, 35, 13029–13042. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. NPJ Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.M.; Shukitt-Hale, B.; Carrihill-Knoll, K.L.; Gomes, S.M. Comparison of the effects of partial- or whole-body exposures to 16O particles on cognitive performance in rats. Radiat. Res. 2014, 181, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Giedzinski, E.; Rola, R.; Fike, J.R.; Limoli, C.L. Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons. Radiat. Res. 2005, 164, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.C.; Craver, B.M.; Tseng, B.P.; Tran, K.K.; Parihar, V.K.; Acharya, M.M.; Limoli, C.L. Mitochondrial-targeted human catalase affords neuroprotection from proton irradiation. Radiat. Res. 2013, 180, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteomics 2011, 74, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Szprengiel, A.; Pluhar, J.; Rabin, B.M.; Joseph, J.A. The effects of proton exposure on neurochemistry and behavior. Adv. Space Res. 2004, 33, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Rusek, A.; Cucinotta, F.A. Issues for simulation of galactic cosmic ray exposures for radiobiological research at ground-based accelerators. Front. Oncol. 2015, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Doherty, A.H.; Genik, P.C.; Gookin, S.E.; Roteliuk, D.M.; Wojda, S.J.; Jiang, Z.S.; McGee-Lawrence, M.E.; Weil, M.M.; Donahue, S.W. Mimicking the effects of spaceflight on bone: Combined effects of disuse and chronic low-dose rate radiation exposure on bone mass in mice. Life Sci. Space Res. 2017, 15, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tahimic, C.G.T.; Globus, R.K. Redox signaling and its impact on skeletal and vascular responses to spaceflight. Int. J. Mol. Sci. 2017, 18, 2153. [Google Scholar] [CrossRef] [PubMed]
- Langlet, C.; Bastide, B.; Canu, M.H. Hindlimb unloading affects cortical motor maps and decreases corticospinal excitability. Exp. Neurol. 2012, 237, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Trinel, D.; Picquet, F.; Bastide, B.; Canu, M.H. Dendritic spine remodeling induced by hindlimb unloading in adult rat sensorimotor cortex. Behav. Brain Res. 2013, 249, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chelyshev, Y.A.; Muhamedshina, Y.O.; Povysheva, T.V.; Shaymardanova, G.F.; Rizvanov, A.A.; Nigmetzyanova, M.V.; Tiapkina, O.V.; Bondarenko, N.I.; Nikolskiy, E.E.; Islamov, R.R. Characterization of spinal cord glial cells in a model of hindlimb unloading in mice. Neuroscience 2014, 280, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Santucci, D.; Kawano, F.; Ohira, T.; Terada, M.; Nakai, N.; Francia, N.; Alleva, E.; Aloe, L.; Ochiai, T.; Cancedda, R.; et al. Evaluation of gene, protein and neurotrophin expression in the brain of mice exposed to space environment for 91 days. PLoS ONE 2012, 7, e40112. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, A.; Cekanaviciute, E.; Smith, D.J.; Costes, S.V. Global transcriptomic analysis suggests carbon dioxide as an environmental stressor in spaceflight: A systems biology GeneLab case study. Sci. Rep. 2018, 8, 4191. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Ramakers, G.M.; Altinbilek, B.; Kas, M.J. Social isolation stress reduces hippocampal long-term potentiation: Effect of animal strain and involvement of glucocorticoid receptors. Neuroscience 2014, 256, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Leser, N.; Wagner, S. The effects of acute social isolation on long-term social recognition memory. Neurobiol. Learn. Mem. 2015, 124, 97–103. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, L.M.; Doran, S.J.; Mwilambwe-Tshilobo, L.; Conti, L.H.; Venna, V.R.; McCullough, L.D. Social isolation after stroke leads to depressive-like behavior and decreased BDNF levels in mice. Behav. Brain Res. 2014, 260, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.R.; Eilertson, K.; Chakraborti, A.; Sharma, S.; Baure, J.; Habdank-Kolaczkowski, J.; Allen, B.; Rosi, S.; Raber, J.; Fike, J.R. Radiation exposure prior to traumatic brain injury induces responses that differ as a function of animal age. Int. J. Radiat. Biol. 2014, 90, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villasana, L.; Rosenberg, J.; Raber, J. Sex-dependent effects of 56Fe irradiation on contextual fear conditioning in C57BL/6J mice. Hippocampus 2010, 20, 19–23. [Google Scholar] [PubMed]
- Britten, R.A.; Mitchell, S.; Johnson, A.M.; Singletary, S.J.; Keeney, S.K.; Nyalwidhe, J.O.; Karbassi, I.D.; Lonart, G.; Sanford, L.D.; Drake, R.R. The identification of serum biomarkers of high-let radiation exposure and biological sequelae. Health Phys. 2010, 98, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Latour, L.; Diaz-Arrastia, R.; Motamedi, V.; Turtzo, C.; Shahim, P.; Mondello, S.; DeVoto, C.; Veras, E.; Hanlon, D.; et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities acutely following a mild traumatic brain injury. Neurology 2018, 91, e1385–e1389. [Google Scholar] [CrossRef] [PubMed]
- Lehallier, B.; Essioux, L.; Gayan, J.; Alexandridis, R.; Nikolcheva, T.; Wyss-Coray, T.; Britschgi, M. Combined plasma and cerebrospinal fluid signature for the prediction of midterm progression from mild cognitive impairment to alzheimer disease. JAMA Neurol. 2016, 73, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 2018, 174, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Mrdjen, D.; Pavlovic, A.; Hartmann, F.J.; Schreiner, B.; Utz, S.G.; Leung, B.P.; Lelios, I.; Heppner, F.L.; Kipnis, J.; Merkler, D.; et al. High-Dimensional Single-Cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 2018, 48, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Nakao-Inoue, H.; Molofsky, A.V.; Oldham, M.C. Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat. Neurosci. 2018, 21, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasca, S.P.; Portmann, T.; Voineagu, I.; Yazawa, M.; Shcheglovitov, A.; Pasca, A.M.; Cord, B.; Palmer, T.D.; Chikahisa, S.; Nishino, S.; et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 2011, 17, 1657–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, K.; Ang, C.E.; Chanda, S.; Olmos, V.H.; Haag, D.; Levinson, D.F.; Sudhof, T.C.; Wernig, M. Transdifferentiation of human adult peripheral blood T cells into neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 6470–6475. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N.; et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; van Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; van Vught, R.; Wilschut, K.J.; Nicolas, A.; Chiang, C.; Lanz, H.L.; Trietsch, S.J.; Joore, J.; Vulto, P. High-throughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Sci. Rep. 2016, 6, 38856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, T.; Sivathanu, V.; Kamm, R.D. Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci. Rep. 2018, 8, 5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics 2016, 13, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Fathali, N.; Doyle, K.P.; Williams, A.M.; Han, J.; Buckwalter, M.S. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia 2014, 62, 1227–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialas, A.R.; Stevens, B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 2013, 16, 1773–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, C.A.; Fleisch, M.C.; Costes, S.V.; Erickson, A.C.; Boissiere, A.; Gupta, R.; Ravani, S.A.; Parvin, B.; Barcellos-Hoff, M.H. Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer Res. 2008, 68, 8304–8311. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, X.; Tang, X.; Wang, P.; Wang, H.; Wang, Y. MiR-21 is continually elevated long-term in the brain after exposure to ionizing radiation. Radiat. Res. 2012, 177, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for enhancing the permeation of CNS-Active drugs through the Blood-Brain Barrier: A review. Molecules 2018, 23, 1289. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothhammer, V.; Kenison, J.E.; Tjon, E.; Takenaka, M.C.; de Lima, K.A.; Borucki, D.M.; Chao, C.C.; Wilz, A.; Blain, M.; Healy, L.; et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, 2012–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutierrez-Vazquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef] [PubMed]


| Targets | Damage | Particle and Dose | Time After Exposure | References |
|---|---|---|---|---|
| Neuronal | Dendritic, axonal and synaptic degeneration | 600 MeV/n 16O: 0.05, 0.3 Gy 600 MeV/n 48Ti: 0.05, 0.3 Gy 600 MeV/n 56Fe: 4 Gy GCR: 0.5 Gy | 3 months 3 months 1, 6, 12 months 4 months | [6,8,32] |
| Neuronal excitability changes | 150 MeV/n protons: 1 Gy | 3 months | [33] | |
| Neuronal proliferation deficits | 300 MeV/n: 0.2, 1 Gy 1 GeV/n 56Fe: 0.3, 1 Gy | 24 h 48 h | [29,30] | |
| Neuronal death | 1 GeV/n 56Fe: 1.6 Gy 500 MeV/n 56Fe: 1.5 Gy | 12 months 1 month | ||
| Glial | Astrocyte activation | 1 GeV/n 56Fe: 1.6 Gy 600 MeV/n 56Fe: 4 Gy | 12 months 1, 6, 12 months | [31,32] |
| Microglial activation | 400 MeV/n 4He: 0.05, 0.3 Gy 400 MeV/n 4He: 0.15, 0.5 Gy 600 MeV/n 16O: 0.05, 0.3 Gy 600 MeV/n 48Ti: 0.05, 0.3 Gy GCR | 12 months 3 months 15, 27 weeks 15, 27 weeks 4 months | [6,8,13,20] | |
| Tissue-level | Oxidative stress | 150 MeV/n protons, 0.5, 2 Gy 1 GeV/n 56Fe, 1.6 Gy 500 MeV/n 56Fe, 1.5 Gy | 1 month 2, 12 months 1 month | [14,17,31] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cekanaviciute, E.; Rosi, S.; Costes, S.V. Central Nervous System Responses to Simulated Galactic Cosmic Rays. Int. J. Mol. Sci. 2018, 19, 3669. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113669
Cekanaviciute E, Rosi S, Costes SV. Central Nervous System Responses to Simulated Galactic Cosmic Rays. International Journal of Molecular Sciences. 2018; 19(11):3669. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113669
Chicago/Turabian StyleCekanaviciute, Egle, Susanna Rosi, and Sylvain V. Costes. 2018. "Central Nervous System Responses to Simulated Galactic Cosmic Rays" International Journal of Molecular Sciences 19, no. 11: 3669. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113669