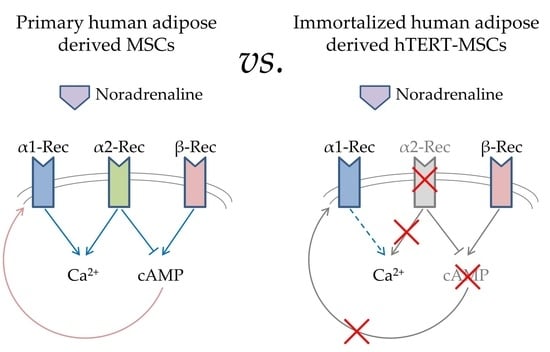
Noradrenaline Sensitivity Is Severely Impaired in Immortalized Adipose-Derived Mesenchymal Stem Cell Line
Abstract
:1. Introduction
2. Results
2.1. Analysis of Adrenergic Receptors Expression in hTERT-MSCs
2.2. Ca2+ Signaling in Single Cells
2.3. Heterologous Sensitization in hTERT-MSCs
2.4. cAMP Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Flow Cytometry
4.3. Immunofluorescence
4.4. Ca2+ Signaling
4.5. cAMP ELISA
4.6. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| adMSCs | Adipose derived mesenchymal stem/stromal cells |
| hTERT | Catalytic subunit of human telomerase |
| hTERT-MSCs | Immortalized by ectopic expression of the catalytic subunit of human telomerase (hTERT) expression adipose-derived MSC |
| IgG | Immunoglobulin G |
| cAMP | Cyclic adenosine monophosphate |
| MFI | Median fluorescence intensity |
References
- Magalon, J.; Daumas, A.; Veran, J.; Magalon, G.; Rossi, P.; Granel, B.; Sabatier, F. Autologous Adipose Tissue-Derived Cells: Are We Talking About Adipose Derived Stem Cells, Stromal Vascular Fraction, or Coleman Fat Grafting? Cell Transplant. 2015, 24, 2667–2668. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Fong, C.; Chen, Y.; Cai, G.; Yang, M. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol. Cell. Endocrinol. 2010, 323, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Kanareikina, M.A.; Kalinina, N.I.; Sysoeva, V.Y.; Dyikanov, D.T.; Stambolsky, D.V.; Tkachuk, V.A. Activation of beta-adrenergic receptors is required for elevated alpha1A-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci. Rep. 2016, 6, 32835. [Google Scholar] [CrossRef] [PubMed]
- Kotova, P.D.; Sysoeva, V.Y.; Rogachevskaja, O.A.; Bystrova, M.F.; Kolesnikova, A.S.; Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Tkachuk, V.A.; Kolesnikov, S.S. Functional expression of adrenoreceptors in mesenchymal stromal cells derived from the human adipose tissue. Biochim. Biophys. Acta 2014, 1843, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Tyurin-Kuzmin, P.A.; Dyikanov, D.T.; Fadeeva, J.I.; Sysoeva, V.Y.; Kalinina, N.I. Flow cytometry analysis of adrenoceptors expression in human adipose-derived mesenchymal stem/stromal cells. Sci. Data 2018, 5, 180196. [Google Scholar] [CrossRef] [PubMed]
- Wolbank, S.; Stadler, G.; Peterbauer, A.; Gillich, A.; Karbiener, M.; Streubel, B.; Wieser, M.; Katinger, H.; van Griensven, M.; Redl, H.; et al. Telomerase immortalized human amnion- and adipose-derived mesenchymal stem cells: Maintenance of differentiation and immunomodulatory characteristics. Tissue Eng. Part A 2009, 15, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Zitnay, J.L.; Reese, S.P.; Tran, G.; Farhang, N.; Bowles, R.D.; Weiss, J.A. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomater. 2018, 65, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Oliver-de La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skladal, P.; Pesl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, S.W.; Park, T.S.; Kim, S.J.; Park, B.C.; Weinstein, D.A.; Lee, Y.M.; Jun, H.S. Aberrant proliferation and differentiation of glycogen storage disease type Ib mesenchymal stem cells. FEBS Lett. 2018, 592, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.U.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Siska, E.K.; Weisman, I.; Romano, J.; Ivics, Z.; Izsvak, Z.; Barkai, U.; Petrakis, S.; Koliakos, G. Generation of an immortalized mesenchymal stem cell line producing a secreted biosensor protein for glucose monitoring. PLoS ONE 2017, 12, e0185498. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, H.; Misawa, H.; Hayashi, T.; Kondo, E.; Yuasa, T.; Kubota, Y.; Seita, M.; Kawamoto, H.; Hassan, W.A.; Hassan, R.A.; et al. Bone repair by transplantation of hTERT-immortalized human mesenchymal stem cells in mice. Transplantation 2009, 88, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Biobank of Lomonosov Moscow State University. Available online: https://human.depo.msu.ru/?d=H/ (accessed on 5 June 2018).





| Primary adMSCs Donor ID | Mean of Responding Cells, % | SD | Relative SD (RSD), % |
|---|---|---|---|
| 1 | 2.03 | 1.56 | 76 |
| 2 | 6.1 | 2.34 | 38 |
| 3 | 2.18 | 0.87 | 39 |
| 4 | 4.56 | 4.56 | 100 |
| 5 | 11.08 | 7.54 | 68 |
| 6 | 3.83 | 2.58 | 67 |
| 7 | 1.86 | 1.63 | 87 |
| 8 | 3.78 | 1.91 | 50 |
| 9 | 18.68 | 15.06 | 80 |
| 10 | 7.82 | 6.52 | 83 |
| 11 | 1.7 | 0.97 | 57 |
| Mean RSD | 68 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyurin-Kuzmin, P.A.; Chechekhin, V.I.; Ivanova, A.M.; Dyikanov, D.T.; Sysoeva, V.Y.; Kalinina, N.I.; Tkachuk, V.A. Noradrenaline Sensitivity Is Severely Impaired in Immortalized Adipose-Derived Mesenchymal Stem Cell Line. Int. J. Mol. Sci. 2018, 19, 3712. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123712
Tyurin-Kuzmin PA, Chechekhin VI, Ivanova AM, Dyikanov DT, Sysoeva VY, Kalinina NI, Tkachuk VA. Noradrenaline Sensitivity Is Severely Impaired in Immortalized Adipose-Derived Mesenchymal Stem Cell Line. International Journal of Molecular Sciences. 2018; 19(12):3712. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123712
Chicago/Turabian StyleTyurin-Kuzmin, Pyotr A., Vadim I. Chechekhin, Anastasiya M. Ivanova, Daniyar T. Dyikanov, Veronika Y. Sysoeva, Natalia I. Kalinina, and Vsevolod A. Tkachuk. 2018. "Noradrenaline Sensitivity Is Severely Impaired in Immortalized Adipose-Derived Mesenchymal Stem Cell Line" International Journal of Molecular Sciences 19, no. 12: 3712. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123712