ZNF521 Represses Osteoblastic Differentiation in Human Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Results
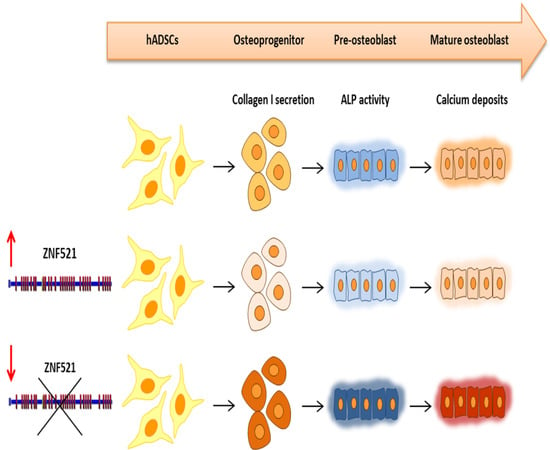
2.1. Ectopic Expression of ZNF521 Reduces Osteoblast Differentiation
2.2. Effect of ZNF521 Knockdown during Osteogenic Differentiation of hADSCs
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transduction
4.2. Osteoblastic Differentiation
4.3. Alkaline Phosphatase Staining
4.4. Alizarin Red S staining
4.5. Immunofluorescence
4.6. Q-RT-PCR
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| hADSCs | Human adipose-derived stem cells |
| ZNF521 | Zinc Finger Protein 521 |
| MSC | Mesenchymal stem cells |
| BMP | Bone morphogenetic protein |
| TGF-β | Transforming growth factor-beta |
| PTH | Parathyroid hormone |
| GH | Growth hormone |
| IGF-1 | Insulin-like growth factor-1 |
| Wnt | Wingless-type MMTV integration site family member |
| Runx2 | Runt-related transcription factor 2 |
| Shh | Sonic Hedgehog |
| Msx2 | Msh homeobox 2 |
| PLZF | Promyelocytic Leukemia zinc-finger protein |
| Osx | Osterix |
| ATF4 | Activating transcription factor 4 |
| ALP | Alkaline phosphatase |
| BCIP/NBT | 5-Bromo-4-chloro-3-indolyl phosphate /nitro blue tetrazolium |
| OPN | Osteopontin |
| HDAC3 | Histone deacetylase 3 |
| bmMSCs | Bone marrow-derived Mesenchymal Stem Cell |
| BSP | Bone sialoprotein |
| OCN | Osteocalcin |
| EBF1 | Early B-cell factor 1 |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Genever, P. Regulation of mesenchymal stem cell differentiation. Adv. Exp. Med. Biol. 2013, 786, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchinato, F.; Karlsson, J.; Ferroni, L.; Gardin, C.; Galli, S.; Wennerberg, A.; Zavan, B.; Andersson, M.; Jimbo, R. Osteogenic potential of human adipose-derived stromal cells on 3-dimensional mesoporous TiO2 coating with magnesium impregnation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.; Le Blanc, K.; Astrom, G.; van Harmelen, V.; Gotherstrom, C.; Blomqvist, L.; Arner, P.; Ryden, M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 2005, 308, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, J.S.; Heller, J.B.; Mitchell, S.A.; Zuk, P.A.; Spoon, D.B.; Wasson, K.L.; Jarrahy, R.; Benhaim, P.; Bradley, J.P. Osteogenic potentiation of human adipose-derived stem cells in a 3-dimensional matrix. Ann. Plast. Surg. 2006, 57, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, G.; Asti, A.; Scaffino, M.F.; Visai, L.; Saino, E.; Cometa, A.M.; Benazzo, F. Human adipose-derived stem cells (hASCs) proliferate and differentiate in osteoblast-like cells on trabecular titanium scaffolds. J. Biomed. Mater. Res. A 2010, 94, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Murakami, H.; Demura, S.; Hayashi, K.; Matsubara, H.; Kato, S.; Yoshioka, K.; Inoue, K.; Ota, T.; Shinmura, K.; et al. A novel method to apply osteogenic potential of adipose derived stem cells in orthopaedic surgery. PLoS ONE 2014, 9, e88874. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J. Osteoblasts: Novel roles in orchestration of skeletal architecture. Int. J. Biochem. Cell Biol. 2003, 35, 1301–1305. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming growth factor-beta1 to the bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.S.; Abou-Samra, A.B. PTH and PTHrP signaling in osteoblasts. Cell Signal. 2009, 21, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mormando, M.; Chiloiro, S.; Bianchi, A.; Giampietro, A.; Angelini, F.; Tartaglione, L.; Nasto, L.; Milardi, D.; Formenti, A.M.; Giustina, A.; et al. Growth hormone receptor isoforms and fracture risk in adult-onset growth hormone-deficient patients. Clin. Endocrinol. 2016, 85, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lough, D.M.; Chambers, C.; Germann, G.; Bueno, R.; Reichensperger, J.; Swanson, E.; Dyer, M.; Cox, L.; Harrison, C.; Neumeister, M.W. Regulation of ADSC Osteoinductive Potential Using Notch Pathway Inhibition and Gene Rescue: A Potential On/Off Switch for Clinical Applications in Bone Formation and Reconstructive Efforts. Plast. Reconstr. Surg. 2016, 138, 642e–652e. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, C.; Li, J.; Liu, L.; Jing, W.; Tang, W.; Tian, W.; Long, J. Effect of miR-26a-5p on the Wnt/Ca(2+) Pathway and Osteogenic Differentiation of Mouse Adipose-Derived Mesenchymal Stem Cells. Calcif. Tissue Int. 2016, 99, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Haxaire, C.; Hay, E.; Geoffroy, V. Runx2 Controls Bone Resorption through the Down-Regulation of the Wnt Pathway in Osteoblasts. Am. J. Pathol. 2016, 186, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Leucht, P.; Levi, B.; Carre, A.L.; Xu, Y.; Helms, J.A.; Longaker, M.T. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng. Part A 2010, 16, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.Q.; Huang, Y.Z.; Chen, X.H.; Xie, H.L.; Zhu, H.M.; Tang, L.; Yang, Z.M.; Huang, Y.C.; Deng, L. Sonic hedgehog enhances the proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Cell Biol. Int. 2012, 36, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shan, S.; Wang, C.; Wang, J.; Li, J.; Hu, G.; Dai, K.; Li, Q.; Zhang, X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp. Cell Res. 2017, 352, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ly, C.; Cipetic, M.; Sims, N.A.; Vieusseux, J.; Kartsogiannis, V.; Bouralexis, S.; Saleh, H.; Zhou, H.; Price, J.T.; et al. Osteoclast inhibitory lectin (OCIL) inhibits osteoblast differentiation and function in vitro. Bone 2007, 40, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Lee, E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Bond, H.M.; Mesuraca, M.; Carbone, E.; Bonelli, P.; Agosti, V.; Amodio, N.; De Rosa, G.; Di Nicola, M.; Gianni, A.M.; Moore, M.A.; et al. Early hematopoietic zinc finger protein (EHZF), the human homolog to mouse Evi3, is highly expressed in primitive human hematopoietic cells. Blood 2004, 103, 2062–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, H.M.; Mesuraca, M.; Amodio, N.; Mega, T.; Agosti, V.; Fanello, D.; Pelaggi, D.; Bullinger, L.; Grieco, M.; Moore, M.A.; et al. Early hematopoietic zinc finger protein-zinc finger protein 521: A candidate regulator of diverse immature cells. Int. J. Biochem. Cell Biol. 2008, 40, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Mega, T.; Lupia, M.; Amodio, N.; Horton, S.J.; Mesuraca, M.; Pelaggi, D.; Agosti, V.; Grieco, M.; Chiarella, E.; Spina, R.; et al. Zinc finger protein 521 antagonizes early B-cell factor 1 and modulates the B-lymphoid differentiation of primary hematopoietic progenitors. Cell Cycle 2011, 10, 2129–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesuraca, M.; Chiarella, E.; Scicchitano, S.; Codispoti, B.; Giordano, M.; Nappo, G.; Bond, H.M.; Morrone, G. ZNF423 and ZNF521: EBF1 Antagonists of Potential Relevance in B-Lymphoid Malignancies. Biomed. Res. Int. 2015, 2015, 165238. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, D.; Banno, S.; Sasai, N.; Ohgushi, M.; Inomata, H.; Watanabe, K.; Kawada, M.; Yakura, R.; Kiyonari, H.; Nakao, K.; et al. Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 2011, 470, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Spina, R.; Filocamo, G.; Iaccino, E.; Scicchitano, S.; Lupia, M.; Chiarella, E.; Mega, T.; Bernaudo, F.; Pelaggi, D.; Mesuraca, M.; et al. Critical role of zinc finger protein 521 in the control of growth, clonogenicity and tumorigenic potential of medulloblastoma cells. Oncotarget 2013, 4, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, E.; Moradi, S.; Nemati, S.; Satarian, L.; Basiri, M.; Gourabi, H.; Zare Mehrjardi, N.; Gunther, P.; Lampert, A.; Handler, K.; et al. Conversion of Human Fibroblasts to Stably Self-Renewing Neural Stem Cells with a Single Zinc-Finger Transcription Factor. Stem Cell Reports 2016, 6, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Pu, J.; Lang, B.; McCaig, C.D. A zinc finger protein Zfp521 directs neural differentiation and beyond. Stem Cell Res. Ther. 2011, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addison, W.N.; Fu, M.M.; Yang, H.X.; Lin, Z.; Nagano, K.; Gori, F.; Baron, R. Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol. Cell. Biol. 2014, 34, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Akerblad, P.; Kiviranta, R.; Gupta, R.K.; Kajimura, S.; Griffin, M.J.; Min, J.; Baron, R.; Rosen, E.D. Regulation of early adipose commitment by Zfp521. PLoS Biol. 2012, 10, e1001433. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.Y.; Lin, S. Zinc finger factor 521 enhances adipogenic differentiation of mouse multipotent cells and human bone marrow mesenchymal stem cells. Oncotarget 2015, 6, 14874–14884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarella, E.; Aloisio, A.; Codispoti, B.; Nappo, G.; Scicchitano, S.; Lucchino, V.; Montalcini, Y.; Camarotti, A.; Galasso, O.; Greco, M.; et al. ZNF521 Has an Inhibitory Effect on the Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hesse, E.; Morvan, F.; Zhang, J.P.; Correa, D.; Rowe, G.C.; Kiviranta, R.; Neff, L.; Philbrick, W.M.; Horne, W.C.; et al. Zfp521 antagonizes Runx2, delays osteoblast differentiation in vitro, and promotes bone formation in vivo. Bone 2009, 44, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, E.; Kiviranta, R.; Wu, M.; Saito, H.; Yamana, K.; Correa, D.; Atfi, A.; Baron, R. Zinc finger protein 521, a new player in bone formation. Ann. N. Y. Acad. Sci. 2010, 1192, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, R.; Yamana, K.; Saito, H.; Ho, D.K.; Laine, J.; Tarkkonen, K.; Nieminen-Pihala, V.; Hesse, E.; Correa, D.; Maatta, J.; et al. Coordinated transcriptional regulation of bone homeostasis by Ebf1 and Zfp521 in both mesenchymal and hematopoietic lineages. J. Exp. Med. 2013, 210, 969–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.T.; Zhan, X.L.; Hu, Z.H. Zinc finger protein 521 suppresses osteogenic differentiation of rat mesenchymal stem cells by inhibiting the Wnt/beta-catenin signaling pathway. Mol. Biol. 2017, 51, 464–472. [Google Scholar] [CrossRef]
- La Rocca, R.; Fulciniti, M.; Lakshmikanth, T.; Mesuraca, M.; Ali, T.H.; Mazzei, V.; Amodio, N.; Catalano, L.; Rotoli, B.; Ouerfelli, O.; et al. Early hematopoietic zinc finger protein prevents tumor cell recognition by natural killer cells. J. Immunol. 2009, 182, 4529–4537. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Saito, H.; Kiviranta, R.; Correa, D.; Yamana, K.; Neff, L.; Toben, D.; Duda, G.; Atfi, A.; Geoffroy, V.; et al. Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity. J. Cell Biol. 2010, 191, 1271–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, D.; Hesse, E.; Seriwatanachai, D.; Kiviranta, R.; Saito, H.; Yamana, K.; Neff, L.; Atfi, A.; Coillard, L.; Sitara, D.; et al. Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev. Cell 2010, 19, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Salerno, L.; Cosentino, C.; Morrone, G.; Amato, F. Computational Modeling of a Transcriptional Switch Underlying B-Lymphocyte Lineage Commitment of Hematopoietic Multipotent Cells. PLoS ONE 2015, 10, e0132208. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, T.; Takei, Y.; Ohmori, R.; Imai, Y.; Ozeki, M.; Tamaki, K.; Haga, H.; Nakamura, T.; Tsuruyama, T. ZFP521 contributes to pre-B-cell lymphomagenesis through modulation of the pre-B-cell receptor signaling pathway. Oncogene 2016, 35, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ge, K. Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci. 2014, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.A.; Akerblad, P.; Sigvardsson, M.; Rosen, E.D. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol. Cell. Biol. 2007, 27, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Kieslinger, M.; Folberth, S.; Dobreva, G.; Dorn, T.; Croci, L.; Erben, R.; Consalez, G.G.; Grosschedl, R. EBF2 regulates osteoblast-dependent differentiation of osteoclasts. Dev. Cell 2005, 9, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, E.; Carra, G.; Scicchitano, S.; Codispoti, B.; Mega, T.; Lupia, M.; Pelaggi, D.; Marafioti, M.G.; Aloisio, A.; Giordano, M.; et al. UMG Lenti: Novel lentiviral vectors for efficient transgene- and reporter gene expression in human early hematopoietic progenitors. PLoS ONE 2014, 9, e114795. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage. Cell Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, B.; Rinaldo, N.; Chiarella, E.; Lupia, M.; Spoleti, C.B.; Marafioti, M.G.; Aloisio, A.; Scicchitano, S.; Giordano, M.; Nappo, G.; et al. Recombinant TAT-BMI-1 fusion protein induces ex vivo expansion of human umbilical cord blood-derived hematopoietic stem cells. Oncotarget 2017, 8, 43782–43798. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarella, E.; Aloisio, A.; Scicchitano, S.; Lucchino, V.; Montalcini, Y.; Galasso, O.; Greco, M.; Gasparini, G.; Mesuraca, M.; Bond, H.M.; et al. ZNF521 Represses Osteoblastic Differentiation in Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 4095. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19124095
Chiarella E, Aloisio A, Scicchitano S, Lucchino V, Montalcini Y, Galasso O, Greco M, Gasparini G, Mesuraca M, Bond HM, et al. ZNF521 Represses Osteoblastic Differentiation in Human Adipose-Derived Stem Cells. International Journal of Molecular Sciences. 2018; 19(12):4095. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19124095
Chicago/Turabian StyleChiarella, Emanuela, Annamaria Aloisio, Stefania Scicchitano, Valeria Lucchino, Ylenia Montalcini, Olimpio Galasso, Manfredi Greco, Giorgio Gasparini, Maria Mesuraca, Heather M. Bond, and et al. 2018. "ZNF521 Represses Osteoblastic Differentiation in Human Adipose-Derived Stem Cells" International Journal of Molecular Sciences 19, no. 12: 4095. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19124095