Immobilization of Denosumab on Titanium Affects Osteoclastogenesis of Human Peripheral Blood Monocytes
Abstract
:1. Introduction
2. Results
2.1. Effect of Soluble Denosumab on Osteoclast Differentiation
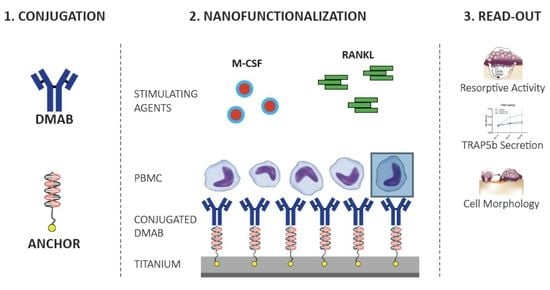
2.2. Nanofunctionalization
2.2.1. TRAP5b Activity
2.2.2. Endogenous Phosphatase Activity
2.2.3. Effect of Immobilized cDMAB on Osteoclast Morphology
3. Discussion
4. Materials and Methods
4.1. Isolation of Peripheral Blood Mononuclear Cells (PBMCs) and Osteoclast Differentiation
4.2. Experimental DMAB Application
4.3. Osteoclast Differentiation: TRAP Histochemistry
4.4. Osteoclast Differentiation: Dentine Resorption Lacunae
4.5. Immobilization of Oligonucleotide Strands on Titanium Specimen
4.6. Hybridization of the Denosumab–ODN Conjugates with Anchored Oligonucleotides
4.7. Quantitative Detection of DMAB
4.8. TRAP Staining and Enzyme-Labeled Fluorescence of Phosphatase Activity
4.9. TRAP5b Secretion
4.10. Scanning Electron Microscopy
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MCSF | Macrophage colony-stimulating factor |
| RANK-L | RANK-Ligand |
| TNF | a Tumor necrosis factor alpha |
| OPG | Osteoprotegerin |
| PBMCs | Peripheral mononuclear cells |
| a-MEM | a-Minimal Essential Medium |
| DMEM | Dulbecco’s Minimal Essential Medium |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
| TRAP | Tartrate-resistant acid phosphatase |
References
- Tsutsumi, R.; Hock, C.; Bechtold, C.D.; Proulx, S.T.; Bukata, S.V.; Ito, H.; Awad, H.A.; Nakamura, T.; O‘Keefe, R.J.; Schwarz, E.M. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. J. Orthop. Res. 2008, 26, 1340–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandelin, J.; Li, T.-F.; Liljeström, M.; Kroon, M.E.; Hanemaaijer, R.; Santavirta, S.; Konttinen, Y.T. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. Bone Jt. J. 2003, 85-B, 1196–1201. [Google Scholar] [CrossRef]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; San Martin, J.; Dansey, R. Bench to bedside: Elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012, 11, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.S.; Kohler, M.I.; Huber, F.; Redeker, J.I.; Schmitt, B.; Schmitt-Sody, M.; Summer, B.; Fottner, A.; Jansson, V.; Mayer-Wagner, S. Factors regulating bone remodeling processes in aseptic implant loosening. J. Orthop. Res. 2017, 35, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, Z.; Xie, J.; Liao, H.; Lv, C.; Hu, Y. Alteration of the RANKL/RANK/OPG System in Periprosthetic Osteolysis with Septic Loosening. Inflammation 2016, 39, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Bajammal, S.; Guyatt, G.H.; Griffith, L.; Busse, J.W.; Schunemann, H.; Einhorn, T.A. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J. Bone Jt. Surg. Am. 2005, 87, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Vinther, M.; Carmody, E.E.; Goater, J.J.; S balle, K.; O‘Keefe, R.J.; Schwarz, E.M. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J. Bone Jt. Surg. Am. 2002, 84-A, 1405–1412. [Google Scholar] [CrossRef]
- Millett, P.J.; Allen, M.J.; Bostrom, M.P. Effects of alendronate on particle-induced osteolysis in a rat model. J. Bone Jt. Surg. Am. 2002, 84-A, 236–249. [Google Scholar] [CrossRef]
- Aspenberg, P.; Agholme, F.; Magnusson, P.; Fahlgren, A. Targeting RANKL for reduction of bone loss around unstable implants: OPG-Fc compared to alendronate in a model for mechanically induced loosening. Bone 2011, 48, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makihira, S.; Mine, Y.; Nikawa, H.; Shuto, T.; Kosaka, E.; Sugiyama, M.; Hosokawa, R. Immobilized-OPG-Fc on a titanium surface inhibits RANKL-dependent osteoclast differentiation in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Mercer, E.; Woo, S.B.; Avorn, J.; Schneeweiss, S.; Treister, N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: Prior work and current challenges. Osteop. Int. 2012, 24, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Suresh, E.; Abrahamsen, B. Denosumab: A novel antiresorptive drug for osteoporosis. Cleve Clin. J. Med. 2015, 82, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Skoldenberg, O.; Rysinska, A.; Eisler, T.; Salemyr, M.; Boden, H.; Muren, O. Denosumab for treating periprosthetic osteolysis; study protocol for a randomized, double-blind, placebo-controlled trial. BMC Musculoskel. Disord. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Rublack, J.; Forster, A.; Schwenzer, B.; Reichert, J.; Scharnweber, D. Functionalization of titanium implants using a modular system for binding and release of VEGF enhances bone implant contact in a rodent model. J. Clin. Periodontol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Strecker, N.; Forster, A.; Schwenzer, B.; Reichert, J.; Scharnweber, D. Angiogenic functionalisation of titanium surfaces using nano-anchored VEGF—An in vitro study. Eur. Cell Mater. 2012, 23, 161–169; discussion 169. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Botel, C.; Forster, A.; Schwenzer, B.; Reichert, J.; Scharnweber, D. Effect of oligonucleotide mediated immobilization of bone morphogenic proteins on titanium surfaces. Biomaterials 2012, 33, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Michael‚, J.; Schönzart, L.; Israel, I.; Beutner, R.; Scharnweber, D.; Worch, H.; Hempel, U.; Schwenzer, B. Oligonucleotide-RGD Peptide Conjugates for Surface Modification of Titanium Implants and Improvement of Osteoblast Adhesion. Bioconj. Chem. 2009, 20, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface R. Soc. 2010, 7 (Suppl. 1), S93–S105. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Förster, A.; Schwenzer, B.; Scharnweber, D. Immobilization of oligonucleotides on titanium based materials by partial incorporation in anodic oxide layers. Biomaterials 2009, 30, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Current Trends in Metallic Orthopedic Biomaterials: From Additive Manufacturing to Bio-Functionalization, Infection Prevention, and Beyond. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Prause, M.; Seeliger, C.; Unger, M.; Rosado Balmayor, E.; van Griensven, M.; Haug, A.T. Pantoprazole Decreases Cell Viability and Function of Human Osteoclasts In Vitro. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Aref, A.; Scharnweber, D.; Bierbaum, S.; Roessler, S.; Sewing, A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin. Oral Implants Res. 2005, 16, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Ferrari, S.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Kostenuik, P.J.; Nguyen, H.Q.; McCabe, J.; Warmington, K.S.; Kurahara, C.; Sun, N.; Chen, C.; Li, L.; Cattley, R.C.; Van, G.; et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 2009, 24, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Ruther, C.; Gabler, C.; Ewald, H.; Ellenrieder, M.; Haenle, M.; Lindner, T.; Mittelmeier, W.; Bader, R.; Kluess, D. In vivo monitoring of implant osseointegration in a rabbit model using acoustic sound analysis. J. Orthop. Res. 2014, 32, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempster, D.W.; Lambing, C.L.; Kostenuik, P.J.; Grauer, A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: A review of preclinical and clinical data. Clin. Ther. 2012, 34, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Sun, Q.; Chen, G.; Sun, S.; Ma, X.; Qiu, H.; Liu, X.; Xu, L.; Liu, M. Jatrorrhizine Hydrochloride Suppresses RANKL-Induced Osteoclastogenesis and Protects against Wear Particle-Induced Osteolysis. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.B.; Stang, E.; Brorson, S.-H.; Andersson, G.; Reinholt, F.P. Tartrate-resistant acid phosphatase (TRAP) co-localizes with receptor activator of NF-KB ligand (RANKL) and osteoprotegerin (OPG) in lysosomal-associated membrane protein 1 (LAMP1)-positive vesicles in rat osteoblasts and osteocytes. Histochem. Cell Biol. 2015, 143, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-Doped Titanium Dioxide Nanotubes Promoted Bone Formation on Titanium Implants. Int. J. Mol. Sci. 2016, 17, 292. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, F.; Hartmann, E.S.; Koehler, M.I.; Redeker, J.I.; Schluessel, S.; Schmitt, B.; Fottner, A.; Unger, M.; van Griensven, M.; Michael, J.; et al. Immobilization of Denosumab on Titanium Affects Osteoclastogenesis of Human Peripheral Blood Monocytes. Int. J. Mol. Sci. 2019, 20, 1002. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051002
Beck F, Hartmann ES, Koehler MI, Redeker JI, Schluessel S, Schmitt B, Fottner A, Unger M, van Griensven M, Michael J, et al. Immobilization of Denosumab on Titanium Affects Osteoclastogenesis of Human Peripheral Blood Monocytes. International Journal of Molecular Sciences. 2019; 20(5):1002. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051002
Chicago/Turabian StyleBeck, Felicitas, Eliza S. Hartmann, Miriam I. Koehler, Julia I. Redeker, Sabine Schluessel, Baerbel Schmitt, Andreas Fottner, Marina Unger, Martijn van Griensven, Jan Michael, and et al. 2019. "Immobilization of Denosumab on Titanium Affects Osteoclastogenesis of Human Peripheral Blood Monocytes" International Journal of Molecular Sciences 20, no. 5: 1002. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051002