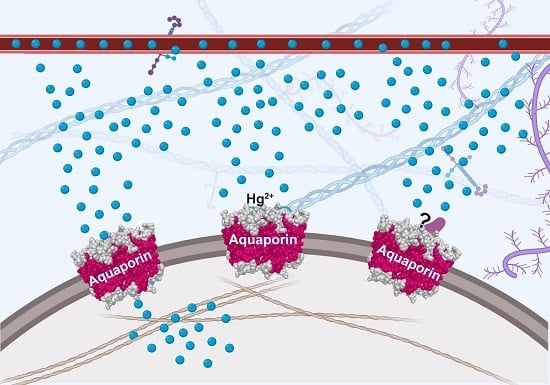
Inhibitors of Mammalian Aquaporin Water Channels
Abstract
:1. Aquaporin Structure
2. Aquaporin Function
2.1. AQP1
2.2. AQP2
2.3. AQP3
2.4. AQP4
2.5. AQPs 0, 5–12
3. Aquaporin Assays
4. Aquaporin Inhibitors
4.1. Small Molecule Inhibitors
4.2. Heavy Metal Ion Inhibitors
5. Antibody Treatments
6. Patents and Clinical Trials
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AQP | aquaporin |
| NPA | Asn-Pro-Ala |
| Å | Ångstrom |
| CNS | central nervous system |
| MIP | major intrinsic protein |
| RNA | ribonucleic acid |
| cRNA | complementary ribonucleic acid |
| MMP | matrix metalloproteinase |
| cAMP | cyclic adenosine monophosphate |
| PKA | protein kinase A |
| NDI | nephrogenic diabetes insipidus |
| KO | knock-out |
| BBB | blood–brain barrier |
| ICP | intracranial pressure |
| NMO | neuromyelitis optica |
| MS | multiple sclerosis |
| AEDs | anti-epileptic drugs |
| miRNA | micro ribonucleic acid |
| siRNA | small interfering ribonucleic acid |
| TEA | tetraethylammonium |
| µM | micromolar |
| HEK293 | human embryonic kidney 293 |
| MCAO | middle cerebral artery occlusion |
| IC50 | half maximal inhibitory concentration |
| NiCl2 | nickel chloride |
| mM | millimolar |
| GFP | green fluorescent protein |
| hAQP | human aquaporin |
| calcein-AM | calcein acetoxymethyl |
| AgNO3 | silver nitrate |
| AgSul | silver sulfadiazine |
| IgG | immunoglobulin G |
| mRNA | messenger ribonucleic acid |
| qPCR | quantitative polymerase chain reaction |
| ELISA | enzyme-linked immunosorbent assay |
| nM | nanomolar |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activate B cells |
| IKK-β | inhibitor of nuclear factor kappa-B |
| 24h | 24 hours |
| TBI | traumatic brain injury |
| mBI | mild brain injury |
| TNF-α | tumor necrosis factor alpha |
| IL1 | interleukin 1 |
| IL6 | interleukin 6 |
| UMIN | University Hospital Medical Information Network |
References
- Simone, L.; Gargano, C.D.; Pisani, F.; Cibelli, A.; Mola, M.G.; Frigeri, A.; Svelto, M.; Nicchia, G.P. Aquaporin-1 inhibition reduces metastatic formation in a mouse model of melanoma. J. Cell. Mol. Med. 2018, 22, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, Y.Z.; Chen, H.; Guo, Z. MicroRNA-488 inhibits proliferation, invasion and EMT in osteosarcoma cell lines by targeting aquaporin 3. Int. J. Oncol. 2018, 53, 1493–1504. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Kageyama, Y.; Tohsaka, A.; Suzuki, F.; Marumo, F.; Sasaki, S. Cloning and Functional Expression of a New Water Channel Abundantly Expressed in the Testis Permeable to Water, Glycerol, and Urea. J. Biol. Chem. 1997, 272, 20782–20786. [Google Scholar] [CrossRef] [Green Version]
- Geyer, R.R.; Musa-Aziz, R.; Qin, X.; Boron, W.F. Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. Am. J. Physiol.-Cell Physiol. 2013, 304, C985–C994. [Google Scholar] [CrossRef]
- Litman, T.; Søgaard, R.; Zeuthen, T. Ammonia and Urea Permeability of Mammalian Aquaporins. In Aquaporins; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 327–358. [Google Scholar] [CrossRef]
- Yang, B.; Verkman, A.S. Water and Glycerol Permeabilities of Aquaporins 1–5 and MIP Determined Quantitatively by Expression of Epitope-tagged Constructs inXenopus Oocytes. J. Biol. Chem. 1997, 272, 16140–16146. [Google Scholar] [CrossRef]
- Agre, P.; Kozono, D. Aquaporin water channels: Molecular mechanisms for human diseases1. FEBS Lett. 2003, 555, 72–78. [Google Scholar] [CrossRef]
- Verkman, A.S.; Smith, A.J.; Phuan, P.-w.; Tradtrantip, L.; Anderson, M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Ther. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [Google Scholar] [CrossRef] [Green Version]
- Castle, N.A. Aquaporins as targets for drug discovery. Drug Discov. Today 2005, 10, 485–493. [Google Scholar] [CrossRef]
- Eriksson, U.K.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom resolution X-ray structure details aquaporin-water interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007, 22, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Tradtrantip, L.; Jin, B.-J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. Adv. Exp. Med. Biol. 2017, 969, 239–250. [Google Scholar] [Green Version]
- Janosi, L.; Ceccarelli, M. The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS ONE 2013, 8, e59897. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Conner, M.T.; Bill, R.M.; Conner, A.C. Structural Determinants of Oligomerization of the Aquaporin-4 Channel. J. Biol. Chem. 2016, 291, 6858–6871. [Google Scholar] [CrossRef]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol.-Ren. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Walz, T. The structure of aquaporins. Quart. Rev. Biophys. 2006, 39, 361–396. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Weinstein, A.M. New Roles for Old Holes: Ion Channel Function in Aquaporin-1. Physiology 2002, 17, 68–72. [Google Scholar] [CrossRef]
- Yool, A.J.; Stamer, W.D.; Regan, J.W. Forskolin Stimulation of Water and Cation Permeability in Aquaporin1 Water Channels. Science 1996, 273, 1216–1218. [Google Scholar] [CrossRef]
- Agre, P.; Lee, M.D.; Devidas, S.; Guggino, W.B. Aquaporins and Ion Conductance. Science 1997, 275, 1490–1492. [Google Scholar] [CrossRef] [Green Version]
- Anthony, T.L.; Brooks, H.L.; Boassa, D.; Leonov, S.; Yanochko, G.M.; Regan, J.W.; Yool, A.J. Cloned Human Aquaporin-1 Is a Cyclic GMP-Gated Ion Channel. Mol. Pharmacol. 2000, 57, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cohen, J.; Boron, W.F.; Schulten, K.; Tajkhorshid, E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J. Struct. Biol. 2007, 157, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Endeward, V.; Arias-Hidalgo, M.; Al-Samir, S.; Gros, G. CO₂ Permeability of Biological Membranes and Role of CO₂ Channels. Membranes 2017, 7, 61. [Google Scholar] [CrossRef]
- Gomes, D.; Agasse, A.; Thiébaud, P.; Delrot, S.; Gerós, H.; Chaumont, F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 1213–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.; Beitz, E.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Characterization of Aquaporin-6 as a Nitrate Channel in Mammalian Cells: REQUIREMENT OF PORE-LINING RESIDUE THREONINE 63. J. Biol. Chem. 2002, 277, 39873–39879. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Kwon, T.-H.; Knepper, M.A.; Nielsen, S.; Agre, P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 1999, 96, 5808–5813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazama, A.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Ion Permeation of AQP6 Water Channel Protein: SINGLE-CHANNEL RECORDINGS AFTER Hg2+ACTIVATION. J. Biol. Chem. 2002, 277, 29224–29230. [Google Scholar] [CrossRef]
- Krane, C.M.; Goldstein, D.L. Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm. Genome 2007, 18, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Saparov, S.M.; Liu, K.; Agre, P.; Pohl, P. Fast and Selective Ammonia Transport by Aquaporin-8. J. Biol. Chem. 2007, 282, 5296–5301. [Google Scholar] [CrossRef]
- Soria, L.R.; Fanelli, E.; Altamura, N.; Svelto, M.; Marinelli, R.A.; Calamita, G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem. Biophys. Res. Commun. 2010, 393, 217–221. [Google Scholar] [CrossRef]
- Horsefield, R.; Nordén, K.; Fellert, M.; Backmark, A.; Törnroth-Horsefield, S.; Terwisscha van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef]
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta (BBA) 2015, 1850, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-G.; Guliaev, A.B.; Walian, P.J.; Jap, B.K. Water Transport in AQP0 Aquaporin: Molecular Dynamics Studies. J. Mol. Biol. 2006, 360, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Chepelinsky, A.B. Structural Function of MIP/Aquaporin 0 in the Eye Lens; Genetic Defects Lead to Congenital Inherited Cataracts. In Aquaporins; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 265–297. [Google Scholar] [CrossRef]
- Harries, W.E.; Akhavan, D.; Miercke, L.J.; Khademi, S.; Stroud, R.M. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 14045–14050. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef]
- Noda, Y.; Sohara, E.; Ohta, E.; Sasaki, S. Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 2010, 6, 168–178. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599. [Google Scholar] [CrossRef]
- Frick, A.; Eriksson, U.K.; de Mattia, F.; Öberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.T.; Törnroth-Horsefield, S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, L.; D’Auria, G.; Vangone, A.; Falcigno, L.; Oliva, R. Structural Basis for Mutations of Human Aquaporins Associated to Genetic Diseases. Int. J. Mol. Sci. 2018, 19, 1577. [Google Scholar] [CrossRef] [PubMed]
- Jeyaseelan, K.; Sepramaniam, S.; Armugam, A.; Wintour, E.M. Aquaporins: A promising target for drug development. Expert Opin. Ther. Targets 2006, 10, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef]
- Soler, D.C.; Young, A.E.; Griffith, A.D.; Fu, P.f.; Cooper, K.D.; McCormick, T.S.; Popkin, D.L. Overexpression of AQP3 and AQP10 in the skin exacerbates psoriasiform acanthosis. Exp. Dermatol. 2017, 26, 949–951. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.C.; Robbins, R.A.; Miercke, L.J.W.; Stroud, R.M. Crystal structure of human aquaporin 4 at 1.8 Å and its mechanism of conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Matsuzaki, T.; Tajika, Y. Aquaporins: Water channel proteins of the cell membrane. Prog. Histochem. Cytochem. 2004, 39, 1–83. [Google Scholar] [CrossRef]
- Yasui, M.; Hazama, A.; Kwon, T.-H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sasaki, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 1997, 1, 247–253. [Google Scholar] [CrossRef]
- Ma, T.; Yang, B.; Verkman, A.S. Cloning of a Novel Water and Urea-Permeable Aquaporin from Mouse Expressed Strongly in Colon, Placenta, Liver, and Heart. Biochem. Biophys. Res. Commun. 1997, 240, 324–328. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwahara, M.; Gu, Y.; Tanaka, Y.; Marumo, F.; Sasaki, S. Cloning and Functional Expression of a New Aquaporin (AQP9) Abundantly Expressed in the Peripheral Leukocytes Permeable to Water and Urea, but Not to Glycerol. Biochem. Biophys. Res. Commun. 1998, 244, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Yoshida, Y.; Tani, T.; Koyama, Y.; Nihei, K.; Ohshiro, K.; Kamiie, J.-I.; Yaoita, E.; Suda, T.; Hatakeyama, K.; et al. Cloning of a New Aquaporin (AQP10) Abundantly Expressed in Duodenum and Jejunum. Biochem. Biophys. Res. Commun. 2001, 287, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Yakata, K.; Tani, K.; Fujiyoshi, Y. Water permeability and characterization of aquaporin-11. J. Struct. Biol. 2011, 174, 315–320. [Google Scholar] [CrossRef]
- Ohta, E.; Itoh, T.; Nemoto, T.; Kumagai, J.; Ko, S.B.H.; Ishibashi, K.; Ohno, M.; Uchida, K.; Ohta, A.; Sohara, E.; et al. Pancreas-specific aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am. J. Physiol.-Cell Physiol. 2009, 297, C1368–C1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, T.; Rai, T.; Kuwahara, M.; Ko, S.B.; Uchida, S.; Sasaki, S.; Ishibashi, K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem. Biophys. Res. Commun. 2005, 330, 832–838. [Google Scholar] [CrossRef]
- Nakano, T.; Nishigami, C.; Irie, K.; Shigemori, Y.; Sano, K.; Yamashita, Y.; Myose, T.; Tominaga, K.; Matsuo, K.; Nakamura, Y.; et al. Goreisan Prevents Brain Edema after Cerebral Ischemic Stroke by Inhibiting Aquaporin 4 Upregulation in Mice. J. Stroke Cerebrovasc. Dis. 2018, 27, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L.S.; Choi, M.; Fernandez, P.C.; Cartron, J.-P.; Agre, P. Defective Urinary Concentrating Ability Due to a Complete Deficiency of Aquaporin-1. N. Engl. J. Med. 2001, 345, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Conner, A.C. Control of the Aquaporin-4 Channel Water Permeability by Structural Dynamics of Aromatic/Arginine Selectivity Filter Residues. Biochemistry 2015, 54, 6753–6755. [Google Scholar] [CrossRef]
- Deen, P.M.T.; van Balkom, B.W.M.; Kamsteeg, E.-J. Routing of the aquaporin-2 water channel in health and disease. Eur. J. Cell Biol. 2000, 79, 523–530. [Google Scholar] [CrossRef]
- Judith Radin, M.; Yu, M.-J.; Stoedkilde, L.; Lance Miller, R.; Hoffert, J.D.; Frokiaer, J.; Pisitkun, T.; Knepper, M.A. Aquaporin-2 regulation in health and disease. Vet. Clin. Pathol. 2012, 41, 455–470. [Google Scholar] [CrossRef]
- Schrier, R.W.; Cadnapaphornchai, M.A. Renal aquaporin water channels: From molecules to human disease. Prog. Biophys. Mol. Biol. 2003, 81, 117–131. [Google Scholar] [CrossRef]
- Brown, D. The ins and outs of aquaporin-2 trafficking. Am. J. Physiol.-Ren. Physiol. 2003, 284, F893–F901. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Prevention of Skin Tumorigenesis and Impairment of Epidermal Cell Proliferation by Targeted Aquaporin-3 Gene Disruption. Mol. Cell. Biol. 2008, 28, 326–332. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Roles of Aquaporin-3 in the Epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 7454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Feng, X.; He, C.; Gao, H.; Yang, L.; Ma, Q.; Guo, L.; Qiao, Y.; Yang, H.; Ma, T. Defective macrophage function in aquaporin-3 deficiency. Faseb J. 2011, 25, 4233–4239. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef]
- Jullienne, A.; Fukuda, A.M.; Ichkova, A.; Nishiyama, N.; Aussudre, J.; Obenaus, A.; Badaut, J. Modulating the water channel AQP4 alters miRNA expression, astrocyte connectivity and water diffusion in the rodent brain. Sci. Rep. 2018, 8, 4186. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Yang, G.-Y. Aquaporin-4: A Potential Therapeutic Target for Cerebral Edema. Int. J. Mol. Sci. 2016, 17, 1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, W.; Jin, H.; Nie, X.; Shen, H.; Li, E.; Wang, W. Curcumin attenuates chronic intermittent hypoxia-induced brain injuries by inhibiting AQP4 and p38 MAPK pathway. Respir. Physiol. Neurobiol. 2018, 255, 50–57. [Google Scholar] [CrossRef]
- Verkman, A.S.; Phuan, P.-W.; Asavapanumas, N.; Tradtrantip, L. Biology of AQP4 and anti-AQP4 antibody: Therapeutic implications for NMO. Brain Pathol. 2013, 23, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Sheilabi, M.A.; Bhattacharyya, D.; Kitchen, P.; Conner, A.C.; Bill, R.M.; Woodroofe, M.N.; Conner, M.T.; Princivalle, A.P. Transcriptome analysis suggests a role for the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. Eur. J. Neurosci. 2017, 46, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Yao, X.; Zador, Z.; Sick, T.J.; Verkman, A.S.; Manley, G.T. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia 2006, 53, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Williamson, A.; Palomba, M.; Eid, T.; de Lanerolle, N.C.; Nagelhus, E.A.; Adams, M.E.; Froehner, S.C.; Agre, P.; Ottersen, O.P. Delayed K+ clearance associated with aquaporin-4 mislocalization: Phenotypic defects in brains of α-syntrophin-null mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13615–13620. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Ischemic brain edema. Prog. Cardiovasc. Dis. 1999, 42, 209–216. [Google Scholar] [CrossRef]
- Simard, J.M.; Kent, T.A.; Chen, M.; Tarasov, K.V.; Gerzanich, V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007, 6, 258–268. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Woodroofe, M.N.; Bill, R.M.; Conner, A.C.; Heath, P.R.; Conner, M.T. Transcriptome Analysis of Gene Expression Provides New Insights into the Effect of Mild Therapeutic Hypothermia on Primary Human Cortical Astrocytes Cultured under Hypoxia. Front. Cell. Neurosci. 2017, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A.; Han, H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Woodroofe, M.N.; Brown, J.E.; Bill, R.M.; Conner, A.C.; Conner, M.T. Hypothermia increases aquaporin 4 (AQP4) plasma membrane abundance in human primary cortical astrocytes via a calcium/transient receptor potential vanilloid 4 (TRPV4)- and calmodulin-mediated mechanism. Eur. J. Neurosci. 2017, 46, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Huber, V.J.; Tsujita, M.; Nakada, T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011, 32, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Treatment of neuromyelitis optica: State-of-the-art and emerging therapies. Nat. Rev. Neurol. 2014, 10, 493. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Zhang, H.; Saadoun, S.; Phuan, P.-W.; Lam, C.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Anti–Aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012, 71, 314–322. [Google Scholar] [CrossRef]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels--from atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta (BBA) 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, J.; Sawa, T.; Yamanaka, N.; Shono, M.; Akamatsu, T.; Tsumura, K.; Parvin, M.N.; Kanamori, N.; Hosoi, K. Involvement of Vesicle–Cytoskeleton Interaction in AQP5 Trafficking in AQP5-Gene-Transfected HSG Cells. Biochem. Biophys. Res. Commun. 1999, 266, 443–447. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine Acts on M3Muscarinic Receptors and Induces the Translocation of Aquaporin5 Water Channel via Cytosolic Ca2+Elevation in Rat Parotid Glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Shimomura, I.; Kishida, K.; Kuriyama, H.; Makino, Y.; Nishizawa, H.; Matsuda, M.; Maeda, N.; Nagaretani, H.; Kihara, S.; et al. Human aquaporin adipose (AQPap) gene. Eur. J. Biochem. 2002, 269, 1814–1826. [Google Scholar] [CrossRef] [Green Version]
- Soveral, G.; Casini, A. Aquaporin modulators: A patent review (2010–2015). Expert Opin. Ther. Patents 2017, 27, 49–62. [Google Scholar] [CrossRef]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar] [CrossRef]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sohara, E.; Kobayashi, K.; Chiga, M.; Rai, T.; Ishibashi, K.; Horie, S.; Su, X.; Zhou, J.; Sasaki, S.; et al. Aberrant Glycosylation and Localization of Polycystin-1 Cause Polycystic Kidney in an AQP11 Knockout Model. J. Am. Soc. Nephrol. 2014, 25, 2789–2799. [Google Scholar] [CrossRef] [Green Version]
- Madeira, A.; Moura, T.F.; Soveral, G. Detecting Aquaporin Function and Regulation. Front. Chem. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Dorr, R.A.; Kierbel, A.; Vera, J.; Parisi, M. A new data-acquisition system for the measurement of the net water flux across epithelia. Comput. Methods Progr. Biomed. 1997, 53, 9–14. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Day, R.E.; Taylor, L.H.; Salman, M.M.; Bill, R.M.; Conner, M.T.; Conner, A.C. Identification and Molecular Mechanisms of the Rapid Tonicity-induced Relocalization of the Aquaporin 4 Channel. J. Biol. Chem. 2015, 290, 16873–16881. [Google Scholar] [CrossRef]
- Patil, R.V.; Xu, S.; van Hoek, A.N.; Rusinko, A.; Feng, Z.; May, J.; Hellberg, M.; Sharif, N.A.; Wax, M.B.; Irigoyen, M.; et al. Rapid Identification of Novel Inhibitors of the Human Aquaporin-1 Water Channel. Chem. Biol. Drug Des. 2016, 87, 794–805. [Google Scholar] [CrossRef] [Green Version]
- Mola, M.G.; Nicchia, G.P.; Svelto, M.; Spray, D.C.; Frigeri, A. Automated cell-based assay for screening of aquaporin inhibitors. Anal. Chem. 2009, 81, 8219–8229. [Google Scholar] [CrossRef]
- Zelenina, M.; Brismar, H. Osmotic water permeability measurements using confocal laser scanning microscopy. Eur. Biophys. J. 2000, 29, 165–171. [Google Scholar] [CrossRef]
- Dickson, P.N.; Margerum, D.W. Extension of accessible first-order rate constants and accurate dead-time determinations for stopped-flow spectroscopy. Anal. Chem. 1986, 58, 3153–3158. [Google Scholar] [CrossRef]
- Keifer, P.A. Flow techniques in NMR spectroscopy. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 62, pp. 1–47. [Google Scholar]
- Hub, J.S.; Grubmüller, H.; de Groot, B.L. Dynamics and Energetics of Permeation Through Aquaporins. What Do We Learn from Molecular Dynamics Simulations? In Aquaporins; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–76. [Google Scholar] [CrossRef]
- Madeira, A.; Camps, M.; Zorzano, A.; Moura, T.F.; Soveral, G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of Aquaporin-1 Water Permeability by Tetraethylammonium: Involvement of the Loop E Pore Region. Mol. Pharmacol. 2000, 57, 1021–1026. [Google Scholar]
- Müller, E.M.; Hub, J.S.; Grubmüller, H.; de Groot, B.L. Is TEA an inhibitor for human Aquaporin-1? Pflug. Archiv Eur. J. Physiol. 2008, 456, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; van Hoek, A.N.; Hediger, M.A. Molecular Characterization of a Broad Selectivity Neutral Solute Channel. J. Biol. Chem. 1998, 273, 24737–24743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Lucks, A.; Gena, P.; Frascaria, D.; Altamura, N.; Svelto, M.; Beitz, E.; Calamita, G. Preparative scale production and functional reconstitution of a human aquaglyceroporin (AQP3) using a cell free expression system. New Biotechnol. 2013, 30, 545–551. [Google Scholar] [CrossRef]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Galán Cobo, A.; Echevarría, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting aquaporin function: Potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef]
- de Almeida, A.; Soveral, G.; Casini, A. Gold compounds as aquaporin inhibitors: New opportunities for therapy and imaging. MedChemComm 2014, 5, 1444–1453. [Google Scholar] [CrossRef]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Alejandra, R.; Natalia, S.; Alicia, E.D. The blocking of aquaporin-3 (AQP3) impairs extravillous trophoblast cell migration. Biochem. Biophys. Res. Commun. 2018, 499, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Zelenina, M.; Bondar, A.A.; Zelenin, S.; Aperia, A. Nickel and extracellular acidification inhibit the water permeability of human aquaporin-3 in lung epithelial cells. J. Biol. Chem. 2003, 278, 30037–30043. [Google Scholar] [CrossRef]
- Ozu, M.; Dorr, R.A.; Teresa Politi, M.; Parisi, M.; Toriano, R. Water flux through human aquaporin 1: Inhibition by intracellular furosemide and maximal response with high osmotic gradients. Eur. Biophys. J. 2011, 40, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tsutomu Nakada, V.H. Inhibitors of Aquaporin 4, Methods and Uses Thereof. U.S. Patent 7,659,312, 9 February 2010. [Google Scholar]
- Pelletier, M.F.; Farr, G.W.; Mcguirk, P.R.; Hall, C.H.; Boron, W.F. Methods of Treating Cerebral Edema. U.S. Patent US9573885B2, 21 February 2017. [Google Scholar]
- Gao, J.; Wang, X.; Chang, Y.; Zhang, J.; Song, Q.; Yu, H.; Li, X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal. Biochem. 2006, 350, 165–170. [Google Scholar] [CrossRef]
- Huber, V.J.; Tsujita, M.; Kwee, I.L.; Nakada, T. Inhibition of Aquaporin 4 by antiepileptic drugs. Bioorg. Med. Chem. 2009, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Biol. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, H.; Verkman, A.S. Lack of aquaporin-4 water transport inhibition by antiepileptics and arylsulfonamides. Bioorg. Med. Chem. 2008, 16, 7489–7493. [Google Scholar] [CrossRef]
- Detmers, F.J.M.; de Groot, B.L.; Müller, E.M.; Hinton, A.; Konings, I.B.M.; Sze, M.; Flitsch, S.L.; Grubmüller, H.; Deen, P.M.T. Quaternary Ammonium Compounds as Water Channel Blockers: SPECIFICITY, POTENCY, AND SITE OF ACTION. J. Biol. Chem. 2006, 281, 14207–14214. [Google Scholar] [CrossRef] [Green Version]
- Søgaard, R.; Zeuthen, T. Test of blockers of AQP1 water permeability by a high-resolution method: No effects of tetraethylammonium ions or acetazolamide. Pflüg. Arch. Eur. J. Physiol. 2008, 456, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Parkkila, S.; Pastorek, J.; Supuran, C.T. Review Article. J. Enzym. Inhib. Med. Chem. 2004, 19, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Yamazaki, M.; Sakimura, K.; Nakada, T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, Y.; Hiroaki, Y.; Fujiyoshi, Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J. Struct. Biol. 2009, 166, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Shank, R.P.; Maryanoff, B.E. Molecular Pharmacodynamics, Clinical Therapeutics, and Pharmacokinetics of Topiramate. CNS Neurosci. Ther. 2008, 14, 120–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirici, I.; Balsanu, T.A.; Bogdan, C.; Margaritescu, C.; Divan, T.; Vitalie, V.; Mogoanta, L.; Pirici, D.; Carare, R.O.; Muresanu, D.F. Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. Int. J. Mol. Sci. 2017, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Nakada, T. Aquaporins in drug discovery and pharmacotherapy. Mol. Asp. Med. 2012, 33, 691–703. [Google Scholar] [CrossRef]
- Esteva-Font, C.; Phuan, P.-W.; Anderson, M.O.; Verkman, A.S. A Small Molecule Screen Identifies Selective Inhibitors of Urea Transporter UT-A. Chem. Biol. 2013, 20, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sasaki, S.; Fushimi, K.; Uchida, S.; Kuwahara, M.; Saito, H.; Furukawa, T.; Nakajima, K.; Yamaguchi, Y.; Gojobori, T. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc. Natl. Acad. Sci. USA 1994, 91, 6269–6273. [Google Scholar] [CrossRef] [PubMed]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins-aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2576–2583. [Google Scholar] [CrossRef] [Green Version]
- Aikman, B.; de Almeida, A.; Meier-Menches, S.M.; Casini, A. Aquaporins in cancer development: Opportunities for bioinorganic chemistry to contribute novel chemical probes and therapeutic agents. Metall. Integr. Biometal Sci. 2018, 10, 696–712. [Google Scholar] [CrossRef]
- Zelenina, M.; Tritto, S.; Bondar, A.A.; Zelenin, S.; Aperia, A. Copper Inhibits the Water and Glycerol Permeability of Aquaporin-3. J. Biol. Chem. 2004, 279, 51939–51943. [Google Scholar] [CrossRef] [Green Version]
- Spinello, A.; de Almeida, A.; Casini, A.; Barone, G. The inhibition of glycerol permeation through aquaglyceroporin-3 induced by mercury(II): A molecular dynamics study. J. Inorg. Biochem. 2016, 160, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Ciancetta, A.; de Almeida, A.; Marrone, A.; Re, N.; Soveral, G.; Casini, A. Aquaporin Inhibition by Gold(III) Compounds: New Insights. ChemMedChem 2013, 8, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; de Almeida, A.; de Graaf, C.; Camps, M.; Zorzano, A.; Moura, T.F.; Casini, A.; Soveral, G. A Gold Coordination Compound as a Chemical Probe to Unravel Aquaporin-7 Function. ChemBioChem 2014, 15, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.V.; Chatterton, J.E.; Sharif, N.A.; Wax, M.B. RNAi-Mediated Inhibition of Aquaporin 4 for Treatment of Ocular Neovascularization. U.S. Patent US20080214486A1, 31 July 2008. [Google Scholar]
- Chatterton, J.E.; Patil, R.V.; Sharif, N.A.; Clark, A.F.; Wax, M.B. RNAi-Mediated Inhibition of Aquaporin 1 for Treatment of Iop-Related Conditions. U.S. Patent US20080171719A1, 4 September 2008. [Google Scholar]
- Haraguchi, G.; Suzuki, J.-I.; Kakuta, T.; Maejima, Y.; Onai, Y.; Isobe, M.; Itai, A.; Fukasawa, H.; Muto, S. Inhibition of IκB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 63, 51–59. [Google Scholar] [Green Version]
- Pelletier, M.F.; Mcguirk, P.R.; Farr, G.W.; Zamboni, R.; Colucci, J.; Zaghdane, H. Prodrug Salts. U.S. Patent US9827253B2, 28 November 2017. [Google Scholar]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.E.; Lindsay, G.; Karina, L.R.; Mary, H.A.; Putnam, J.; Eliceiri, B.; Coimbra, R.; Bansal, V. Ghrelin decreases motor deficits after traumatic brain injury. J. Surg. Res. 2014, 187, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V. Methods of Treating Mild Brain Injury. U.S. Patent US10105416B2, 23 October 2018. [Google Scholar]
- Hossienzadeh, F.; Babri, S.; Alipour, M.R.; Ebrahimi, H.; Mohaddes, G. Effect of ghrelin on brain edema induced by acute and chronic systemic hypoxia. Neurosci. Lett. 2013, 534, 47–51. [Google Scholar] [CrossRef]
- Lopez, N.E.; Krzyzaniak, M.J.; Blow, C.; Putnam, J.; Ortiz-Pomales, Y.; Hageny, A.-M.; Eliceiri, B.; Coimbra, R.; Bansal, V. Ghrelin Prevents Disruption of the Blood–Brain Barrier after Traumatic Brain Injury. J. Neurotrauma 2012, 29, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M. Highly Soluble Aquaporin-4 Extracellular Loop c Peptide Immunization for Treatment of Neuromyelitis Optica. U.S. Patent US20170080063A1, 23 March 2017. [Google Scholar]
- Flynn, G.A.; Yool, A.J.; Migliati, E.R.; Ritter, L.S. Aquaporin Modulators and Methods of Using Them for the Treatment of Edema and Fluid Imbalance. U.S. Patent US20150224108A1, 13 August 2015. [Google Scholar]
- Bar-shavit, Z.; Aharon, R. Modulation of Osteoclast Differentiation. U.S. Patent US20150065427A1, 5 March 2015. [Google Scholar]

| AQP | Structural Information Available | Water Permeability (Glycerol Permeability Is Also Given for Aquaglyceroporins) | Main Locations in Humans | Disease States | References |
|---|---|---|---|---|---|
| AQP0 | PDB ref: 1YMG Resolution: 2.2 Å Species: Bos taurus Method: X-ray diffraction Amino acids: 263 | Xenopus oocytes—5 ng cRNA Pf: 1.3 × 10−3 cm/s (control with no AQP 0.67 × 10−3 cm/s) pf: 0.25 × 10−14 cm3/s—single channel permeability | Lens | Mutations in the gene for AQP0 often result in bilateral cataracts | [7,34,35,36] |
| AQP1 | PDB ref: 1FQY Resolution: 3.8 Å Species: Homo sapiens Method: Electron crystallography Amino acids: 269 | Xenopus oocytes—5 ng cRNA Pf: 19 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 6.0 × 10−14 cm3/s—single channel permeability | Erythrocytes Choroid plexus Eye epithelium Corneal epithelium Bile duct Capillaries Nephron Airways Skeletal muscle | Deficiency causes inability to concentrate urine Tumors with AQP1 have increased metastases and invasiveness Altered pain sensation | [3,7,37,38,39,40] |
| AQP2 | PDB ref: 4NEF Resolution: 2.75 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 242 | Xenopus oocytes—5 ng cRNA Pf: 10 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 3.3 × 10−14 cm3/s—single channel permeability | Collecting duct cells | Deficiency causes diabetes insipidus | [7,39,41,42] |
| AQP3 |
No structural data available UniProtKB—O92482 Species: Homo sapiens Amino acids: 292 | Xenopus oocytes—5 ng cRNA Pf: 8.0 × 10−3 cm/s (control 0.67 × 10c3 cm/s) pf: 2.1 × 10−14 cm3/s—single channel permeability Pgly: 23 × 10−6 cm/s | Epidermis Collecting duct cells Erythrocytes | Skin dehydration Psoriasis Tumor invasiveness and metastases | [7,43,44,45] |
| AQP4 | PDB ref: 3GD8 Resolution: 1.8 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 223 | Xenopus oocytes—5 ng cRNA Pf: 29 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 24 × 10−14 cm3/s—single channel permeability | Astrocytes Parietal cells Collecting duct cells Retina | Cytotoxic edema Vasogenic edema Neuromyelitis optica | [7,44,46] |
| AQP5 | PDB ref: 3D9S Resolution: 2 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 266 | Xenopus oocytes—5 ng cRNA Pf: 10 × 10−3 cm/s (control 0.67 × 10−3 cm/s) pf: 5.0 × 10−14 cm3/s—single channel permeability | Salivary, lacrimal and sweat glands Lung | Sjögren’s syndrome | [7,32,47] |
| AQP6 |
No structural data available UniProtKB—Q13520 Species: Homo sapiens Amino acids: 282 | Xenopus oocytes—5–10 ng cRNA Pf: 1.2 × 10−3 cm/s (control 0.53 × 10−3 cm/s) (93 × 10 −4 cm/s after activation with mercury) No single channel data available. | Collecting duct | Unknown | [48,49] |
| AQP7 |
No structural data available UniProtKB—O14520 Species: Homo sapiens Amino acids: 342 | Xenopus oocytes—5 ng cRNA Pf: 18.6 × 10−3 cm/s (control 1.7 × 10−3 cm/s) Pgly: 18.9 × 10−6 cm/s No single channel data available | Adipocytes Testis Proximal kidney tubule | Adipocyte hypertrophy | [4,8] |
| AQP8 |
No structural data available UniProtKB—O94778 Species: Homo sapiens Amino acids: 261 | Xenopus oocytes—10 ng cRNA Pf: 22 × 10−3 cm/s (control 0.8 × 10−3 cm/s) pf: 8.2 × 10−14 cm3/s—single channel permeability | Pancreas Testis | Unknown | [44,50] |
| AQP9 | No structural data available UniProtKB—O43315 Species: Homo sapiens Amino acids: 295 | Xenopus oocytes—10 ng cRNA Pf: 12.3 × 10−3 cm/s (control 1.8 × 10−3 cm/s) Pgly: ~10 × 10−6 cm/s (controls: H2O 0 cm/s, AQP4 ~2.5 × 10−6 cm/s) No single channel data available | Hepatocytes Osteoclasts Astrocytes Leukocytes | Osteoporosis | [43,51] |
| AQP10 | PDB ref: 6F7H Resolution: 2.3 Å Species: Homo sapiens Method: X-ray diffraction Amino acids: 301 | Xenopus oocytes—10 ng cRNA Pf: ~0.05 × 10−3 cm/s (controls: negative control ~0.02 × 10−3 cm/s, AQP8 ~0.2 × 10−3 cm/s No single channel data available | Intestinal epithelial cells Adipocytes | Unknown | [44,52] |
| AQP11 |
No structural data available UniProtKB—Q8NBQ7 Species: Homo sapiens Amino acids: 271 | Spodoptera frugiperda—Sf9 membrane vesicles containing 1 µg mouse AQP11 protein Pf: 29.8 × 10−3 cm/s (controls: negative control 8.2 × 10−3 cm/s, 246.1 × 10−3 cm/s human AQP1, 197.7 × 10−3 cm/s His-rat AQP4) No single channel data available | Testis Thymus Kidney Liver | Polycystic kidneys | [44,53] |
| AQP12 |
No structural data available AQP12A UniProtKB—Q8IXF9 AQP12B UniProtKB—A6NM10 Species: Homo sapiens Amino acids: 295 | Pancreatic rough endoplasmic reticulum vesicles—600 µg total RNA Pf: 13.0 × 10−3 cm/s (control 15.1 × 10−3 cm/s) non-significant difference No single channel data available | Pancreatic acinar cells | Unknown | [54,55] |
| Assay Type | Assay Principle | Benefits | Limitations | References |
|---|---|---|---|---|
| Epithelial | Epithelial cells are placed on supports, solutes are added and transepithelial flux is measured by the height of the fluid in capillary tubes | Assists AQP identification and characterization in tissues Individual membrane permeability is calculated | Not very robust Measures paracellular as well as cellular water flow | [94,95] |
| Osmotic Swelling | Osmotic gradients are used to cause flux of solutes and water in cells endogenously-expressing or transfected with AQP genes; cell volume changes are measured | Well-established technique Oocytes have low intrinsic water permeability Other cells can be used Inward and outward gradients possible | Preparation techniques vary causing discrepancies in results between laboratories Not very reproducible Variability between oocytes | [94,96,98] |
| Microscopy | Most often fluorescent dyes are used that permeate the plasma membrane and are cleaved by esterases. As osmotic shock occurs, the fluorescence is altered, which is measured and used to calculate cell volume changes | Linear relationship produced between fluorophore and cell volume changes More accurate than counterparts Very sensitive Robust | Thickness of cell-line monolayer can produce variability in results Fluorophore may not always stay within the cell depending on cell-line | [94,99,104] |
| Stopped-Flow Spectroscopy | Suspensions of cells or vesicles are mixed with various osmotic solutions and flux of water causes volume changes, which result in altered light scattering. The linear relationship between optical properties and cell volume allows permeability to be calculated; alternatively fluorophores can be used. | Fast kinetics measured Can use cells, vesicles and proteoliposomes Linear relationship between optical properties and volume changes | Variability in dead time and mixing ability can cause problems with reproducibility | [94,99,101] |
| Computational Methods | High resolution atomic structures of AQPs are used and molecular dynamics simulations allow in silico measurement of water permeability | Provides new insights into AQP structure and function | High-quality structures not always available May disagree with experimental data | [94,103] |
| Inhibitor | Conditions | AQPs Inhibited | Structure | References |
|---|---|---|---|---|
| Tetraethyl-ammonium | Xenopus oocytes 100 µM | hAQP1 |  | [105,106] |
| Phloretin | Xenopus oocytes 0.1 mM Proteoliposomes 0.5 mM | rAQP9 hAQP3 |  | [107,108] |
| Mercury chloride | Xenopus oocytes 1 mM | hAQP1 |  | [96,109] |
| AuPhen | Erythrocytes 50 µM Adipocytes 15 µM | hAQP3 hAQP7 |  | [110,111] |
| Silver nitrate | Erythrocytes 10 µM | hAQP3 |  | [112] |
| Copper sulfate | Swan 71 cells 100 µM | hAQP3 |  | [113] |
| Nickel chloride | Human bronchial epithelium cells 1 mM | hAQP3 |  | [114] |
| Furosemide | Xenopus oocytes 10 µM | hAQP1 |  | [115] |
| Bumetanide | Xenopus oocytes 100 µM | rAQP4 |  | [116] |
| N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl) acetamide | Xenopus oocytes 20 µM | hAQP4-M23 |  | [117] |
| IMD-0354 | Mice 0.76 mg/kg | mAQP4-M23 |  | [118] |
| Acetazolamide | HEK293 cells 10 µM | rAQP1 rAQP4 |  | [119] |
| TGN-020 | C57/BL6 male mice 200 mg/kg (23–28 g) | mAQP4 |  | [81] |
| Topiramate | Xenopus oocytes 20 µM | rAQP4-M23 |  | [120] |
| DFP00173 | Human erythrocytes 25 µM | hAQP3 |  | [121] |
| Z433927330 | Human erythrocytes 25 µM | mAQP7 |  | [121] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.T.; Conner, A.C.; Bill, R.M. Inhibitors of Mammalian Aquaporin Water Channels. Int. J. Mol. Sci. 2019, 20, 1589. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071589
Abir-Awan M, Kitchen P, Salman MM, Conner MT, Conner AC, Bill RM. Inhibitors of Mammalian Aquaporin Water Channels. International Journal of Molecular Sciences. 2019; 20(7):1589. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071589
Chicago/Turabian StyleAbir-Awan, Mohammed, Philip Kitchen, Mootaz M. Salman, Matthew T. Conner, Alex C. Conner, and Roslyn M. Bill. 2019. "Inhibitors of Mammalian Aquaporin Water Channels" International Journal of Molecular Sciences 20, no. 7: 1589. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20071589