Characterization of a Surface-Active Protein Extracted from a Marine Strain of Penicillium chrysogenum
Abstract
:1. Introduction
2. Results
2.1. Purification and Identification of the Penicillium chrysogenum BE Protein
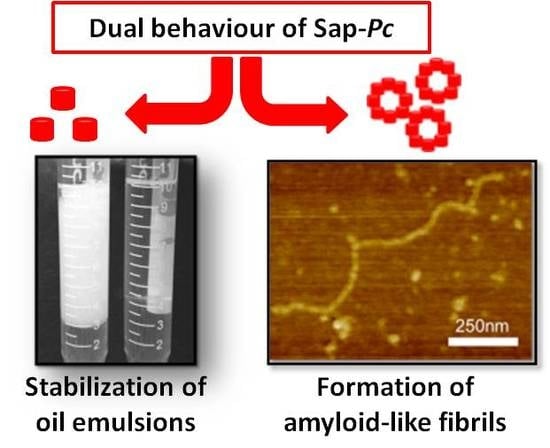
2.2. Protein Characterization as Biosurfactant
2.3. SAP-Pc Aggregation
2.4. Effect of Protein Concentration on SAP-Pc Aggregation
2.5. Toxicity Analysis
3. Discussion
4. Materials and Methods
4.1. Culture Conditions and Protein Extraction
4.2. Mass Spectrometry
4.3. Emulsification Index
4.4. Spectroscopy Techniques
4.5. AFM
4.6. Surface Tension Measurements
4.7. Dynamic Light Scattering
4.8. Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| BE | Bioemulsifier |
| BS | Biosurfactant |
| cac | Critical aggregation concentration |
| CD | Circular dichroism |
| cmc | Critical micellar concentration |
| DLS | Dynamic light scattering |
| E24 | Emulsification index |
| FTIR | Fourier transform infrared spectroscopy |
| HPB | Hydrophobin |
| LC–MSMS | Liquid chromatography tandem mass spectrometry |
| MALDI–TOF | Matrix assisted laser desorption ionization–time of flight |
| SDS–PAGE | Sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| TCA | Trichloroacetic acid |
| ThT | Thioflavin T |
| TLC | Thin layer chromatography |
References
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.M.; Wadhawa, G.; Banat, I.M.; Chopade, B.A. Mapping of patents on bioemulsifier and biosurfactant: A review. J. Sci. Ind. Res. 2006, 65, 91–115. [Google Scholar]
- Araújo, H.W.C.; Andrade, R.F.S.; Montero-Rodríguez, D.; Rubio-Ribeaux, D.; Alves da Silva, C.A.; Campos-Takaki, G.M. Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microb. Cell Fact. 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Rufino, R.; Luna, J.; Santos, V.; Sarubbo, L. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental Applications of Biosurfactants: Recent Advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef]
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]
- Harayama, S.; Kishira, H.; Kasai, Y.; Shutsubo, K. Petroleum biodegradation in marine environments. J. Mol. Microbiol. Biotechnol. 1999, 1, 63–70. [Google Scholar]
- Michel, J.P.; Rosales, A. Marine Bioremediation—A Sustainable Biotechnology of Petroleum Hydrocarbons Biodegradation in Coastal and Marine Environments. J. Bioremediation Biodegrad. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Raghukumar, C. Marine fungal biotechnology: An ecological perspective. Fungal Divers. 2008, 31, 19–35. [Google Scholar]
- Imhoff, J.; Imhoff, F.J. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, G.; Cameotra, S.S.; Chopra, H.K. Biosurfactants from Fungi: A Review. J. Pet. Environ. Biotechnol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Bovio, E.; Gnavi, G.; Prigione, V.; Spina, F.; Denaro, R.; Yakimov, M.; Calogero, R.; Crisafi, F.; Varese, G.C. The culturable mycobiota of a Mediterranean marine site after an oil spill: Isolation, identification and potential application in bioremediation. Sci. Total Environ. 2017, 576, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.; Teixeira, J.; Rodrigues, L.; Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants Produced by Marine Microorganisms with Therapeutic Applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.P. Hydrophobins-unique fungal proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Berger, B.W.; Sallada, N.D. Hydrophobins: Multifunctional biosurfactants for interface engineering. J. Biol. Eng. 2019, 13, 10. [Google Scholar] [CrossRef]
- Cox, P.W.; Hooley, P. Hydrophobins: New prospects for biotechnology. Fungal Biol. Rev. 2009, 23, 40–47. [Google Scholar] [CrossRef]
- Khalesi, M.; Gebruers, K.; Derdelinckx, G. Recent Advances in Fungal Hydrophobin Towards Using in Industry. Protein J. 2015, 34, 243–255. [Google Scholar] [CrossRef]
- Ren, Q.; Kwan, A.H.; Sunde, M. Two forms and two faces, multiple states and multiple uses: Properties and applications of the self-assembling fungal hydrophobins. Biopolymers 2013, 100, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Cicatiello, P.; Gravagnuolo, A.M.; Gnavi, G.; Varese, G.C.; Giardina, P. Marine fungi as source of new hydrophobins. Int. J. Biol. Macromol. 2016, 92, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Cicatiello, P.; Dardano, P.; Pirozzi, M.; Gravagnuolo, A.M.; De Stefano, L.; Giardina, P. Self-assembly of two hydrophobins from marine fungi affected by interaction with surfaces. Biotechnol. Bioeng. 2017, 10, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, J.; Kohlmeyer, E. Marine Mycology: The Higher Fungi; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Huang, J.; Lu, C.; Qian, X.; Huang, Y.; Zheng, Z.; Shen, Y. Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol. Sin. 2011, 30, 118–123. [Google Scholar] [CrossRef]
- Blesic, M.; Dichiarante, V.; Milani, R.; Linder, M.; Metrangolo, P. Conference paper Evaluating the potential of natural surfactants in the petroleum industry: The case of hydrophobins. Pure Appl. Chem. 2018, 90, 305–314. [Google Scholar] [CrossRef]
- Askolin, S.; Linder, M.; Scholtmeijer, K.; Tenkanen, M.; Penttilä, M.; de Vocht, M.L.A.; Wösten, H.A.B. Interaction and Comparison of a Class I Hydrophobin from Schizophyllum commune and Class II Hydrophobins from Trichoderma reesei. Biomacromolecules 2006, 7, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Szilvay, G.R.; Kisko, K.; Serimaa, R.; Linder, M.B. The relation between solution association and surface activity of the hydrophobin HFBI from Trichoderma reesei. FEBS Lett. 2007, 581, 2721–2726. [Google Scholar] [CrossRef]
- Arosio, P.; Knowles, T.P.J.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [Green Version]
- Fung, S.Y.; Keyes, C.; Duhamel, J.; Chen, P. Concentration effect on the aggregation of a self-assembling oligopeptide. Biophys. J. 2003, 85, 537–548. [Google Scholar] [CrossRef]
- Novo, M.; Freire, S.; Al-Soufi, W. Critical aggregation concentration for the formation of early Amyloid-β (1–42) oligomers. Sci. Rep. 2018, 8, 1783. [Google Scholar] [CrossRef]
- Kroes-Nijboer, A.; Venema, P.; Bouman, J.; van der Linden, E. The Critical Aggregation Concentration of β-Lactoglobulin-Based Fibril Formation. Food Biophys. 2009, 4, 59–63. [Google Scholar] [CrossRef]
- Parmar, A.S.; Muschol, M. Hydration and hydrodynamic interactions of lysozyme: effects of chaotropic versus kosmotropic ions. Biophys. J. 2009, 97, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Menger, F.M.; Rizvi, S.A.A. Relationship between Surface Tension and Surface Coverage. Langmuir 2011, 27, 13975–13977. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Lomakin, A.; Kirkitadze, M.D.; Teplow, D.B.; Chen, S.-H.; Benedek, G.B. Structure determination of micelle-like intermediates in amyloid beta -protein fibril assembly by using small angle neutron scattering. Proc. Natl. Acad. Sci. USA 2002, 99, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Dear, A.J.; Šarić, A.; Michaels, T.C.T.; Dobson, C.M.; Knowles, T.P.J. Statistical Mechanics of Globular Oligomer Formation by Protein Molecules. J. Phys. Chem. B 2018, 122, 11721–11730. [Google Scholar] [CrossRef] [PubMed]
- Itri, F.; Monti, D.M.; Della Ventura, B.; Vinciguerra, R.; Chino, M.; Gesuele, F.; Lombardi, A.; Velotta, R.; Altucci, C.; Birolo, L.; et al. Femtosecond UV-laser pulses to unveil protein–protein interactions in living cells. Cell. Mol. Life Sci. 2016, 73, 637–648. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Pinto, B.; Nici, F.; Dardano, P.; Rea, I.; De Stefano, L.; Mayol, L.; Piccialli, G.; Borbone, N. Self-Assembly of G-Rich Oligonucleotides Incorporating a 3′-3′ Inversion of Polarity Site: A New Route Towards G-Wire DNA Nanostructures. ChemistryOpen 2017, 6, 599–605. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Imperatore, R.; De Santis, A.; Luchini, A.; Paduano, L.; D’Errico, G. Structure and dynamics of cetyltrimethylammonium chloride-sodium dodecylsulfate (CTAC-SDS) catanionic vesicles: High-value nano-vehicles from low-cost surfactants. J. Colloid Interface Sci. 2017, 501, 112–122. [Google Scholar] [CrossRef]
- Arciello, A.; De Marco, N.; Del Giudice, R.; Guglielmi, F.; Pucci, P.; Relini, A.; Monti, D.M.; Piccoli, R. Insights into the fate of the N-terminal amyloidogenic polypeptide of ApoA-I in cultured target cells. J. Cell. Mol. Med. 2011, 15, 2652–2663. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicatiello, P.; Stanzione, I.; Dardano, P.; De Stefano, L.; Birolo, L.; De Chiaro, A.; Monti, D.M.; Petruk, G.; D’Errico, G.; Giardina, P. Characterization of a Surface-Active Protein Extracted from a Marine Strain of Penicillium chrysogenum. Int. J. Mol. Sci. 2019, 20, 3242. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133242
Cicatiello P, Stanzione I, Dardano P, De Stefano L, Birolo L, De Chiaro A, Monti DM, Petruk G, D’Errico G, Giardina P. Characterization of a Surface-Active Protein Extracted from a Marine Strain of Penicillium chrysogenum. International Journal of Molecular Sciences. 2019; 20(13):3242. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133242
Chicago/Turabian StyleCicatiello, Paola, Ilaria Stanzione, Principia Dardano, Luca De Stefano, Leila Birolo, Addolorata De Chiaro, Daria Maria Monti, Ganna Petruk, Gerardino D’Errico, and Paola Giardina. 2019. "Characterization of a Surface-Active Protein Extracted from a Marine Strain of Penicillium chrysogenum" International Journal of Molecular Sciences 20, no. 13: 3242. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133242