Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells
Abstract
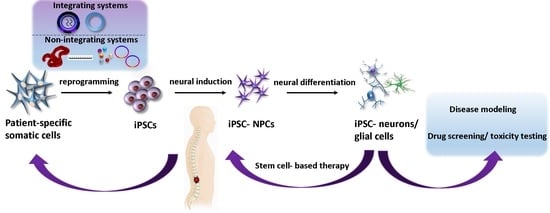
:1. Introduction
2. Differentiation of iPSCs into NPCs
3. Advances in Scaffold Construction
4. SCI Modeling and Therapy with iPSC-Derived NPCs
5. Important Considerations before Clinical Application of iPSCs-NPCs in SCI
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khazaei, M.; Siddiqui, A.; Fehlings, M. The Potential for iPS-Derived Stem Cells as a Therapeutic Strategy for Spinal Cord Injury: Opportunities and Challenges. JCM 2014, 4, 37–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzekou, A.; Fehlings, M.G. Treatment of Spinal Cord Injury with Intravenous Immunoglobulin G: Preliminary Evidence and Future Perspectives. J. Clin. Immunol. 2014, 34, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, M.S.; Li, Q.; Bresnahan, J.C. Cell death and plasticity after experimental spinal cord injury. Prog. Brain Res. 2000, 128, 9–21. [Google Scholar] [PubMed]
- Salewski, R.P.F.; Eftekharpour, E.; Fehlings, M.G. Are induced pluripotent stem cells the future of cell-based regenerative therapies for spinal cord injury? J. Cell. Physiol. 2009, 222, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Ahuja, C.S.; Fehlings, M.G. Induced Pluripotent Stem Cells for Traumatic Spinal Cord Injury. Front. Cell Dev. Biol. 2017, 4, 152. [Google Scholar] [CrossRef] [PubMed]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Young, W.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J.; et al. A Randomized, Controlled Trial of Methylprednisolone or Naloxone in the Treatment of Acute Spinal-Cord Injury: Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990, 322, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Wilson, J.R.; Cho, N. Methylprednisolone for the Treatment of Acute Spinal Cord Injury: Counterpoint. Neurosurgery 2014, 61, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okano, H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Moreno Manzano, V.; Stojkovic, M.; Bhattacharya, S.S.; Erceg, S. Concise Review: Human Pluripotent Stem Cells in the Treatment of Spinal Cord Injury. Stem Cells 2012, 30, 1787–1792. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Stem cells in human neurodegenerative disorders—Time for clinical translation? J. Clin. Invest. 2010, 120, 29–40. [Google Scholar] [CrossRef]
- Ronaghi, M.; Erceg, S.; Moreno-Manzano, V.; Stojkovic, M. Challenges of Stem Cell Therapy for Spinal Cord Injury: Human Embryonic Stem Cells, Endogenous Neural Stem Cells or Induced Pluripotent Stem Cells? Stem Cells 2009, 28, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Matsumoto, A.; Shimazaki, T.; Enoki, R.; Koizumi, A.; Ishii, S.; Itoyama, Y.; Sobue, G.; Okano, H. Spatiotemporal Recapitulation of Central Nervous System Development by Murine Embryonic Stem Cell-Derived Neural Stem/Progenitor Cells. Stem Cells 2008, 26, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S. Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Okano, H. Applications of induced pluripotent stem cell technologies in spinal cord injury. J. Neurochem. 2017, 141, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transpl. 2017, 26, 585–603. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Grabel, L. Directing the differentiation of embryonic stem cells to neural stem cells. Dev. Dyn. 2007, 236, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.W.; Croft, G.F.; Williams, D.J.; O’Keeffe, S.; Carrasco, M.A.; Davis, A.R.; Roybon, L.; Oakley, D.H.; Maniatis, T.; Henderson, C.E.; et al. Accelerated High-Yield Generation of Limb-Innervating Motor Neurons from Human Stem Cells. J. Neurosci. 2013, 33, 574–586. [Google Scholar] [CrossRef]
- Nutt, S.E.; Chang, E.-A.; Suhr, S.T.; Schlosser, L.O.; Mondello, S.E.; Moritz, C.T.; Cibelli, J.B.; Horner, P.J. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp. Neurol. 2013, 248, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Lukovic, D.; Diez Lloret, A.; Stojkovic, P.; Rodríguez-Martínez, D.; Perez Arago, M.A.; Rodriguez-Jimenez, F.J.; González-Rodríguez, P.; López-Barneo, J.; Sykova, E.; Jendelova, P.; et al. Highly Efficient Neural Conversion of Human Pluripotent Stem Cells in Adherent and Animal-Free Conditions: Pluripotent Stem Cell Derived Neural Progenitors. Stem Cells Transl. Med. 2017, 6, 1217–1226. [Google Scholar] [CrossRef]
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, D.R.; Kim, H.-S.; Yoo, J.-E.; Jung, S.J.; Lim, B.Y.; Jang, J.; Kang, H.-C.; You, S.; Hwang, D.-Y.; et al. Highly Pure and Expandable PSA-NCAM-Positive Neural Precursors from Human ESC and iPSC-Derived Neural Rosettes. PLoS ONE 2012, 7, e39715. [Google Scholar] [CrossRef] [PubMed]
- Badja, C.; Maleeva, G.; El-Yazidi, C.; Barruet, E.; Lasserre, M.; Tropel, P.; Binetruy, B.; Bregestovski, P.; Magdinier, F. Efficient and Cost-Effective Generation of Mature Neurons From Human Induced Pluripotent Stem Cells: Neuronal Differentiation From hiPSCs. Stem Cells Transl. Med. 2014, 3, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, L.L.; Wang, Y.C.; He, X.; Jiang, L.; Fu, S.J.; Han, X.F.; Liu, J.; Wang, T.H. Oligodendrocyte precursor cell transplantation promotes functional recovery following contusive spinal cord injury in rats and is associated with altered microRNA expression. Mol. Med. Rep. 2018, 17, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hashimoto, M.; Koda, M.; Naito, A.T.; Murata, A.; Okawa, A.; Takahashi, K.; Yamazaki, M. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell–derived astrocytes in a rat spinal cord injury model. SPI 2011, 15, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Avci, H.X.; Ochalek, A.; Rösingh, L.N.; Molnár, K.; László, L.; Bellák, T.; Téglási, A.; Pesti, K.; Mike, A.; et al. Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2017, 25, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; Marro, S.; Patzke, C.; Acuna, C.; Covy, J.; et al. Rapid Single-Step Induction of Functional Neurons from Human Pluripotent Stem Cells. Neuron 2013, 78, 785–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compagnucci, C.; Nizzardo, M.; Corti, S.; Zanni, G.; Bertini, E. In vitro neurogenesis: Development and functional implications of iPSC technology. Cell. Mol. Life Sci. 2014, 71, 1623–1639. [Google Scholar] [CrossRef]
- Choi, H.W.; Hong, Y.J.; Kim, J.S.; Song, H.; Cho, S.G.; Bae, H.; Kim, C.; Byun, S.J.; Do, J.T. In vivo differentiation of induced pluripotent stem cells into neural stem cells by chimera formation. PLoS ONE 2017, 12, e0170735. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, O.; Miura, K.; Okada, Y.; Fujiyoshi, K.; Mukaino, M.; Nagoshi, N.; Kitamura, K.; Kumagai, G.; Nishino, M.; Tomisato, S.; et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 12704–12709. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, Y.; Abematsu, M.; Falk, A.; Tsujimura, K.; Sanosaka, T.; Juliandi, B.; Semi, K.; Namihira, M.; Komiya, S.; Smith, A.; et al. Treatment of a Mouse Model of Spinal Cord Injury by Transplantation of Human Induced Pluripotent Stem Cell-Derived Long-Term Self-Renewing Neuroepithelial-Like Stem Cells. Stem Cells 2012, 30, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Okada, Y.; Itakura, G.; Iwai, H.; Nishimura, S.; Yasuda, A.; Nori, S.; Hikishima, K.; Konomi, T.; Fujiyoshi, K.; et al. Pre-Evaluated Safe Human iPSC-Derived Neural Stem Cells Promote Functional Recovery after Spinal Cord Injury in Common Marmoset without Tumorigenicity. PLoS ONE 2012, 7, e52787. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itakura, G.; Kobayashi, Y.; Nishimura, S.; Iwai, H.; Takano, M.; Iwanami, A.; Toyama, Y.; Okano, H.; Nakamura, M. Controlling Immune Rejection Is a Fail-Safe System against Potential Tumorigenicity after Human iPSC-Derived Neural Stem Cell Transplantation. PLoS ONE 2015, 10, e0116413. [Google Scholar] [CrossRef] [PubMed]
- Romanyuk, N.; Amemori, T.; Turnovcova, K.; Prochazka, P.; Onteniente, B.; Sykova, E.; Jendelova, P. Beneficial Effect of Human Induced Pluripotent Stem Cell-Derived Neural Precursors in Spinal Cord Injury Repair. Cell Transpl. 2015, 24, 1781–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okubo, T.; Iwanami, A.; Kohyama, J.; Itakura, G.; Kawabata, S.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Matsubayashi, K.; et al. Pretreatment with a γ-Secretase Inhibitor Prevents Tumor-like Overgrowth in Human iPSC-Derived Transplants for Spinal Cord Injury. Stem Cell Rep. 2016, 7, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, Y.; Pang, M.; Yang, Y.; Li, S.; Liu, L.; Shu, T.; Zhou, W.; Wang, X.; Rong, L.; et al. Tissue-engineered regeneration of completely transected spinal cord using induced neural stem cells and gelatin-electrospun poly (lactide-co-glycolide)/polyethylene glycol scaffolds. PLoS ONE 2015, 24, e0117709. [Google Scholar] [CrossRef] [PubMed]
- Mohtaram, N.K.; Ko, J.; King, C.; Sun, L.; Muller, N.; Jun, M.B.; Willerth, S.M. Electrospun biomaterial scaffolds with varied topographies for neuronal differentiation of human-induced pluripotent stem cells. J. Biomed. Mater. Res. A 2015, 103, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shi, G.; Fan, B.; Cheng, X.; Zhang, X.; Wang, X.; Liu, S.; Hao, Y.; Wei, Z.; Wang, L.; et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int. J. Nanomed. 2018, 10, 6265–6277. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, C.; Chen, X.; Zou, Y.; Zhou, Z.; Lin, C.; Tan, G.; Zhou, L.; Ning, C.; Wang, Q. Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2018, 30, 17742–17755. [Google Scholar] [CrossRef]
- Agbay, A.; Edgar, J.M.; Robinson, M.; Styan, T.; Wilson, K.; Schroll, J.; Ko, J.; Khadem Mohtaram, N.; Jun, M.B.; Willerth, S.M. Biomaterial Strategies for Delivering Stem Cells as a Treatment for Spinal Cord Injury. Cells Tissues Organs 2016, 202, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Murgoci, A.-N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal Cord Injury: Animal Models, Imaging Tools and the Treatment Strategies. Neurochem. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Guo, Y.; Yang, D.-G.; Yang, M.-L.; Du, L.-J.; Li, J.-J. Induced Pluripotent Stem Cell Transplantation Improves Locomotor Recovery in Rat Models of Spinal Cord Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cell. Physiol. Biochem. 2018, 47, 1835–1852. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Yamanaka, S. iPS cell technologies: Significance and applications to CNS regeneration and disease. Mol. Brain 2014, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Rybárová, S.; Vecanová, J.; Hodorová, I.; Mihalik, J.; Čižmáriková, M.; Mojžiš, J.; Solár, P.; Benický, M.; Adamkov, M.; Mirossay, L. Association between polymorphisms of XRCC1, p53 and MDR1 genes, the expression of their protein products and prognostic significance in human breast cancer. Med. Sci. Monit. 2011, 17, BR354–BR363. [Google Scholar] [CrossRef] [PubMed]
- Adamkov, M.; Lauko, L.; Balentova, S.; Pec, J.; Pec, M.; Rajcani, J. Expression pattern of anti-apoptotic protein survivin in dysplastic nevi. Neoplasma 2009, 56, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ahuja, C.S.; Salewski, R.P.; Li, L.; Satkunendrarajah, K.; Nagoshi, N.; Shibata, S.; Fehlings, M.G. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS ONE 2017, 12, e0182339. [Google Scholar] [CrossRef]
- Okubo, T.; Nagoshi, N.; Kohyama, J.; Tsuji, O.; Shinozaki, M.; Shibata, S.; Kase, Y.; Matsumoto, M.; Nakamura, M.; Okano, H. Treatment with a Gamma-Secretase Inhibitor Promotes Functional Recovery in Human iPSC- Derived Transplants for Chronic Spinal Cord Injury. Stem Cell Rep. 2018, 11, 1416–1432. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Pereira, I.M.; Marote, A.; Salgado, A.J.; Silva, N.A. Filling the gap: Neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals 2019, 12, 65. [Google Scholar] [CrossRef]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, K.; Nagoshi, N.; Tsuji, O.; Matsumoto, M.; Okano, H.; Nakamura, M. Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment. Int. J. Mol. Sci. 2019, 20, 1054. [Google Scholar] [CrossRef] [PubMed]
- López-Serrano, C.; Torres-Espín, A.; Hernández, J.; Alvarez-Palomo, A.B.; Requena, J.; Gasull, X.; Edel, M.J.; Navarro, X. Effects of the Post-Spinal Cord Injury Microenvironment on the Differentiation Capacity of Human Neural Stem Cells Derived from Induced Pluripotent Stem Cells. Cell Transpl. 2016, 25, 1833–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Animal Model | Lesion Type | Lesion Site | Starting Cell Type | Obtained Cells After Neural Induction | Differentiated Cell Types for TP | Timing of TP After SCI | Outcome after TP and Time of Recovery | References |
|---|---|---|---|---|---|---|---|---|
| C57BL/6N adult mouse | Contusion injury by IH impactor | T10 | Mouse iPSCs | iPSC- derived neurospheres | iPSC-NPCs | 9 days | Functional recovery – 21 d.; inhibition of astrogliosis, no teratoma formation | [29] |
| NOD/SCID mouse | Contusion injury by IH impactor | T10 | Mouse iPSCs | iPSC- derived neurospheres | iPSC-NPCs | 9 days | Functional recovery – 42 d.; no tumor formation | [30] |
| NOD/SCID mouse | Contusion injury by IH impactor | T9-T10 | Mouse iPSCs | iPSC- derived neurospheres | iPSC- astrocytes | 3–7 days | No functional recovery; increase of sensitivity to mechanical stimuli | [24] |
| NOD/SCID mouse | Contusion injury | T10 | Human iPSCs | iPSC- derived neurospheres | hiPSC-NPCs | 7 days | Functional recovery – 8 w., graft survival with incomplete filling of lesions | [31] |
| Common marmoset | Contusion injury by weight-drop device | C5 | Human iPSCs | iPSC- derived neurospheres | hiPSC- NSCs | 9 days | Functional recovery – 56 d., angiogenesis, remyelinization; no tumor formation | [32] |
| Long- Evans rat | Hemi-contusion by Ohio State Injury Device | C4 | Human iPSCs | iPSC- derived neural tube rosettes | hiPSCs - NPCs; iPSC-OPs | 4 weeks | No functional improvement; graft survival with incomplete filling of lesion | [18] |
| Athymic nude rat | Lateral hemi-section | C5 | Human iPSCs | iPSC- derived neural tube rosettes | hiPSC-NSCs | 2 weeks | No functional recovery; robust extension of axons without myelination; | [33] |
| BALB/cA mouse | Laminectomy | T10 | Human iPSCs | iPSC- derived neurospheres | Tumorigenic hiPSC-NSC/NPCs | daily (28 days) | Massive rejection of hiPSC-NSC/NPC – based tumors cause by cessation of immunosuppressants | [34] |
| Wistar rats | Balloon induced-compression | T8-T9 | Human iPSCs | iPSC- derived EBs | iPSC-NPCs | 7 days | Functional recovery – 14 d.; differentiated neurons, oligodendrocytes, astrocytes; axonal regrowth | [35] |
| NOD/SCID mouse | Contusion injury | T10 | Human iPSCs | iPSC- derived neurospheres | OPs- derived from hiPSC-NPCs | 9 days | Improvement of functional recovery- 35 d.; injured axons remyelination; no tumor formation | [20] |
| Wistar rats | Balloon induced-compression | T10 | Human iPSCs | iPSC- derived neurospheres | iPSC-NPCs | 7 days | Functional recovery – 8 w.; reduced astrogliosis; decrease inflammation | [15] |
| Wistar rats | Contusion injury | T9-T11 | Mouse iPSCs | iPSC- derived EBs | OPs- derived from iPSC-NPCs | 7 days | Possible promotion of functional recovery based on the results of a miRNA assay – 7 d. | [23] |
| NOD/SCID mouse | Contusion injury | T10 | Human iPSCs | iPSC- derived EBs | hiPSC-NSC/NPCs | 9 days | Improvement of locomotor function; axonal regrowth and remyelination – 42 d.; no tumor formation | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csobonyeiova, M.; Polak, S.; Zamborsky, R.; Danisovic, L. Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2019, 20, 3838. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20153838
Csobonyeiova M, Polak S, Zamborsky R, Danisovic L. Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells. International Journal of Molecular Sciences. 2019; 20(15):3838. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20153838
Chicago/Turabian StyleCsobonyeiova, Maria, Stefan Polak, Radoslav Zamborsky, and Lubos Danisovic. 2019. "Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells" International Journal of Molecular Sciences 20, no. 15: 3838. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20153838