Glomerular Collagen Deposition and Lipocalin-2 Expression Are Early Signs of Renal Injury in Prediabetic Obese Rats
Abstract
:1. Introduction
2. Results
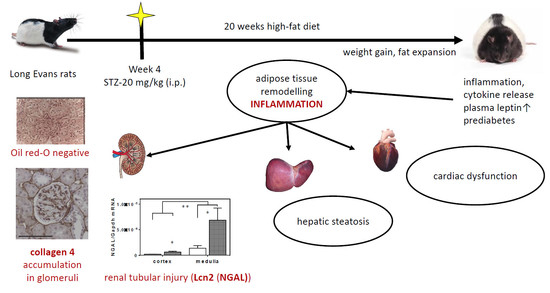
2.1. Twenty Weeks of HFD with a Single Low-Dose of STZ Induced Obesity with Prediabetes, Adipose Tissue Remodelling but Preserved Liver Function in Long-Evans Rats
2.2. Renal Function was Preserved but Significant Glomerular Collagen Deposition and Tubular Lcn2 Expression Appeared in PRED Rats
2.3. Known mRNA and miRNA Markers of Renal Fibrosis were Unaffected in PRED Rats
2.4. mRNA Expression of Inflammatory, Oxidative Stress, and Metabolic Markers in the Kidney in PRED Rats
2.5. Phosphorylation of Akt on Ser473 was Reduced in PRED Rat Livers
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Animal Model and Experimental Setup
4.1.1. Ethics Statement
4.1.2. Animal Model
4.1.3. Renal Sample Collection
4.2. Analysis of Functional Kidney Parameters
4.3. Analysis of Renal Morphology
4.4. Analysis of Plasma Lipid and Functional Liver Parameters
4.5. Immunohistochemistry
4.6. Gene-Expression Analysis of the Renal and Adipose Tissue Samples
4.6.1. RNA Preparation
4.6.2. Quantitative Real-Time PCR Analysis
4.6.3. Messenger RNA Detection
4.6.4. microRNA Detection:
4.7. Western Blot of Liver Lysates
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
References
- Kovesdy, C.P.; Furth, S.; Zoccali, C. Obesity and kidney disease: Hidden consequences of the epidemic. Physiol. Int. 2017, 104, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Horvath, B.; Rajesh, M.; Varga, Z.V.; Gariani, K.; Ryu, D.; Cao, Z.; Holovac, E.; Park, O.; Zhou, Z.; et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 2017, 66, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenta, I.; Varga, Z.V.; Valentine, H.; Cinar, R.; Horti, A.; Mathews, W.B.; Dannals, R.F.; Steele, K.; Kunos, G.; Wahl, R.L.; et al. Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: A translational approach. JACC Cardiovasc. Imaging 2018, 11, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kelly, A.S. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, C.; Tatsis, I.; Thomopoulos, C.; Wilcox, C.; Palm, F.; Kordalis, A.; Katsiki, N.; Papademetriou, V.; Stefanadis, C. Effects of hypertension, diabetes mellitus, obesity and other factors on kidney haemodynamics. Curr. Vasc. Pharmacol. 2014, 12, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Erdelyi, K.; Paloczi, J.; Cinar, R.; Zsengeller, Z.K.; Jourdan, T.; Matyas, C.; Nemeth, B.T.; Guillot, A.; Xiang, X.; et al. Disruption of renal arginine metabolism promotes kidney injury in hepatorenal syndrome in mice. Hepatology 2018, 68, 1519–1533. [Google Scholar] [CrossRef]
- Cildir, G.; Akincilar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: All immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Monteillet, L.; Gjorgjieva, M.; Silva, M.; Verzieux, V.; Imikirene, L.; Duchampt, A.; Guillou, H.; Mithieux, G.; Rajas, F. Intracellular lipids are an independent cause of liver injury and chronic kidney disease in non alcoholic fatty liver disease-like context. Mol. Metab. 2018, 16, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Koncsos, G.; Varga, Z.V.; Baranyai, T.; Boengler, K.; Rohrbach, S.; Li, L.; Schluter, K.D.; Schreckenberg, R.; Radovits, T.; Olah, A.; et al. Diastolic dysfunction in prediabetic male rats: Role of mitochondrial oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H927–H943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Decleves, A.E.; Zolkipli, Z.; Satriano, J.; Wang, L.; Nakayama, T.; Rogac, M.; Le, T.P.; Nortier, J.L.; Farquhar, M.G.; Naviaux, R.K.; et al. Regulation of lipid accumulation by AMP-activated kinase corrected in high fat diet-induced kidney injury. Kidney Int. 2014, 85, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Ericksen, M.B.; Hamano, Y.; Neilson, E.G.; Ziyadeh, F.; Kalluri, R. Differential expression of type IV collagen isoforms in rat glomerular endothelial and mesangial cells. Biochem. Biophys. Res. Commun. 2002, 295, 401–407. [Google Scholar] [CrossRef]
- Tang, F.; Hao, Y.; Zhang, X.; Qin, J. Effect of echinacoside on kidney fibrosis by inhibition of TGF-beta1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des. Dev. Ther. 2017, 11, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Xia, L.; Goldberg, H.J.; Lee, K.W.; Shah, A.; Stavar, L.; Masson, E.A.; Momen, A.; Shikatani, E.A.; John, R.; et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 2013, 62, 3874–3886. [Google Scholar] [CrossRef]
- Brenner, B.M.; Lawler, E.V.; Mackenzie, H.S. The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int. 1996, 49, 1774–1777. [Google Scholar] [CrossRef] [Green Version]
- Ruggenenti, P.; Porrini, E.L.; Gaspari, F.; Motterlini, N.; Cannata, A.; Carrara, F.; Cella, C.; Ferrari, S.; Stucchi, N.; Parvanova, A.; et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012, 35, 2061–2068. [Google Scholar] [CrossRef]
- Matsusaka, T.; Sandgren, E.; Shintani, A.; Kon, V.; Pastan, I.; Fogo, A.B.; Ichikawa, I. Podocyte injury damages other podocytes. JASN 2011, 22, 1275–1285. [Google Scholar] [CrossRef]
- Barber, T.; Vina, J.R.; Vina, J.; Cabo, J. Decreased urea synthesis in cafeteria-diet-induced obesity in the rat. Biochem. J. 1985, 230, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabater, D.; Agnelli, S.; Arriaran, S.; Fernandez-Lopez, J.A.; Romero Mdel, M.; Alemany, M.; Remesar, X. Altered nitrogen balance and decreased urea excretion in male rats fed cafeteria diet are related to arginine availability. Biomed. Res. Int. 2014, 2014, 959420. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Wang, Z. The update of NGAL in Acute kidney injury. Curr. Protein Peptide Sci. 2017, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kaucsar, T.; Godo, M.; Revesz, C.; Kovacs, M.; Mocsai, A.; Kiss, N.; Albert, M.; Krenacs, T.; Szenasi, G.; Hamar, P. Urine/Plasma neutrophil gelatinase associated lipocalin ratio is a sensitive and specific marker of subclinical acute kidney injury in mice. PLoS ONE 2016, 11, e0148043. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.D.; Pitashny, M.; Gindea, S.; Tieng, A.T.; Levine, B.; Goilav, B.; Campbell, S.R.; Xia, Y.; Qing, X.; Thomas, D.B.; et al. Neutrophil gelatinase-associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis in mice. Arthritis Rheum. 2012, 64, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Viau, A.; El Karoui, K.; Laouari, D.; Burtin, M.; Nguyen, C.; Mori, K.; Pillebout, E.; Berger, T.; Mak, T.W.; Knebelmann, B.; et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Investig. 2010, 120, 4065–4076. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Ichinose, K.; Maeshima, Y.; Yamamoto, Y.; Kinomura, M.; Hirokoshi, K.; Kitayama, H.; Takazawa, Y.; Sugiyama, H.; Yamasaki, Y.; Agata, N.; et al. 2-(8-hydroxy-6-methoxy-1-oxo-1h-2-benzopyran-3-yl) propionic acid, an inhibitor of angiogenesis, ameliorates renal alterations in obese type 2 diabetic mice. Diabetes 2006, 55, 1232–1242. [Google Scholar] [CrossRef]
- Borgeson, E.; Johnson, A.M.; Lee, Y.S.; Till, A.; Syed, G.H.; Ali-Shah, S.T.; Guiry, P.J.; Dalli, J.; Colas, R.A.; Serhan, C.N.; et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 2015, 22, 125–137. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, X.Y.; Eirin, A.; Woollard, J.R.; Jordan, K.L.; Tang, H.; Lerman, A.; Lerman, L.O. Perirenal fat promotes renal arterial endothelial dysfunction in obese swine through tumor necrosis factor-alpha. J. Urol. 2016, 195, 1152–1159. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Ziyadeh, F.N. Leptin and renal fibrosis. Contrib. Nephrol. 2006, 151, 175–183. [Google Scholar] [PubMed]
- Wolf, G.; Chen, S.; Han, D.C.; Ziyadeh, F.N. Leptin and renal disease. Am. J. Kidney Dis. 2002, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Hamann, A.; Han, D.C.; Helmchen, U.; Thaiss, F.; Ziyadeh, F.N.; Stahl, R.A. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: Potential role in glomerulosclerosis. Kidney Int. 1999, 56, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Maimaitiyiming, H.; Qi, X.; Norman, H.; Wang, S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am. J. Physiol. Renal Physiol. 2013, 305, F871–F880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-Derived oleanolic acid (OA) ameliorates risk factors of cardiovascular diseases in a diet-induced pre-diabetic rat model: Effects on selected cardiovascular risk factors. Molecules 2019, 24, 340. [Google Scholar] [CrossRef]
- Johari, T.Y.; Ghoneim, M.A.; Moselhy, S.S. Thyroid profile and LDH Isoenzymes as prognostic biomarkers for diabetic and/or obese subjects. Afr. Health Sci. 2018, 18, 697–706. [Google Scholar] [CrossRef]
- Tateyama, Y.; Techasrivichien, T.; Musumari, P.M.; Suguimoto, S.P.; Zulu, R.; Macwan’gi, M.; Dube, C.; Ono-Kihara, M.; Kihara, M. Obesity matters but is not perceived: A cross-sectional study on cardiovascular disease risk factors among a population-based probability sample in rural Zambia. PLoS ONE 2018, 13, e0208176. [Google Scholar] [CrossRef]
- Mena-Sanchez, G.; Babio, N.; Martinez-Gonzalez, M.A.; Corella, D.; Schroder, H.; Vioque, J.; Romaguera, D.; Martinez, J.A.; Lopez-Miranda, J.; Estruch, R.; et al. Fermented dairy products, diet quality, and cardio-metabolic profile of a Mediterranean cohort at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1002–1011. [Google Scholar] [CrossRef]
- Sepandar, F.; Rashidbeygi, E.; Maghbooli, Z.; Khorrami-Nezhad, L.; Hajizadehoghaz, M.; Mirzaei, K. The association between resting metabolic rate and metabolic syndrome May Be mediated by adipokines in overweight and obese women. Diabetes Metab. Syndr. 2019, 13, 530–534. [Google Scholar] [CrossRef]
- Habib, S.S.; Bashir, S.; Iqbal, M.; Abdelaziz, G.M.; Alyahya, R.; Alzahrani, G.K.; Alangari, S.I.; Alrayes, N.A.; Alkahtani, D.S.; Alonso-Alonso, M. Cardiovascular risk and neurocognitive assessment in young adults and their relationship to body adiposity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7929–7935. [Google Scholar] [CrossRef] [PubMed]
- Keskek, S.O.; Kursun, O.; Ortoglu, G.; Bankir, M.; Tuzun, Z.; Saler, T. Obesity without comorbidity may also lead to non-thyroidal illness syndrome. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.P.; Rabelink, T.J. A possible role of cystatin C in adipose tissue homeostasis may impact kidney function estimation in metabolic syndrome. Nephrol. Dial. Transplant. 2013, 28, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Garcia, C.; Izquierdo-Lahuerta, A.; Vivas, Y.; Velasco, I.; Yeo, T.K.; Chen, S.; Medina-Gomez, G. Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS ONE 2015, 10, e0142291. [Google Scholar] [CrossRef] [PubMed]
- Udi, S.; Hinden, L.; Earley, B.; Drori, A.; Reuveni, N.; Hadar, R.; Cinar, R.; Nemirovski, A.; Tam, J. Proximal tubular cannabinoid-1 receptor regulates obesity-induced CKD. J. Am. Soc. Nephrol. JASN. 2017, 28, 3518–3532. [Google Scholar] [CrossRef] [PubMed]
- Mansor, L.S.; Gonzalez, E.R.; Cole, M.A.; Tyler, D.J.; Beeson, J.H.; Clarke, K.; Carr, C.A.; Heather, L.C. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovasc. Diabetol. 2013, 12, 136. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 536–537. [Google Scholar]
- Liu, Q.; Nilsen-Hamilton, M. Identification of a new acute phase protein. J. Biol. Chem. 1995, 270, 22565–22570. [Google Scholar] [CrossRef]








| Species | Gene Symbol | Primer Direction | Sequence (5′->3′) |
|---|---|---|---|
| rno | Adpn | fwd | 5′-AAAGGAGAGCCTGGAGAAGC-3′ |
| rev | 5′-GTCCCGGAATGTTGCAGTAG-3′ | ||
| rno | Gapdh | fwd | 5′-AACTTGCCGTGGGTAGAG-3′ |
| rev | 5′-ATGGTGAAGGTCGGTGTG-3′ | ||
| rno | IL-1β | fwd | 5′-AGA GTG TGG ATC CCA AAC AA -3′ |
| rev | 5′-AGT CAA CTA TGT CCC GAC CA -3′ | ||
| rno | Lcn2 | fwd | 5′-GATGTTGTTATCCTTGAGGCCC-3′ |
| rev | 5′-CACTGACTACGACCAGTTTGCC-3′ | ||
| rno | Nrf2 | fwd | 5′-CTC TCT GGA GAC GGC CAT GAC T-3′ |
| rev | 5′-CTG GGC TGG GGA CAG TGG TAG T-3′ | ||
| rno | Ob-Ra/532 | fwd | 5′-GACGATGTTCCAAACCCCAAG-3′ |
| rev | 5′-TGGGAGGTTGGTAGATTGGATTC-3′ | ||
| rno | PPARγ | fwd | 5′-CTGCCTATGAGCACTTCACAAG-3′, |
| rev | 5′-ATCACGGAGAGGTCCACAGA-3′ | ||
| rno | TGF-β1 | fwd | 5′-AGCCCTGTATTCCGTCTCCT-3′ |
| rev | 5′-ATTCCTGGCGTTACCTTGG-3′ | ||
| rno | TNFα | fwd | 5′-TTCTCATTCCTGCTCGTGGC-3′ |
| rev | 5′-AACTGATGAGAGGGAGCCCA-3′ | ||
| rno | HSP90B | fwd | 5′-GGAAGCCCCCGCCCTCTGTATA-3′ |
| rev | 5′-AGGGCCAGTCAAGGCTGTTGG-3′ | ||
| rno | 28S | fwd | 5′-GGTAAACGGCGGGAGTAACT-3′ |
| rev | 5′-TCACCGTGCCAGACTAGAGT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukosza, E.N.; Kaucsár, T.; Godó, M.; Lajtár, E.; Tod, P.; Koncsos, G.; Varga, Z.V.; Baranyai, T.; Tu Nguyen, M.; Schachner, H.; et al. Glomerular Collagen Deposition and Lipocalin-2 Expression Are Early Signs of Renal Injury in Prediabetic Obese Rats. Int. J. Mol. Sci. 2019, 20, 4266. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174266
Bukosza EN, Kaucsár T, Godó M, Lajtár E, Tod P, Koncsos G, Varga ZV, Baranyai T, Tu Nguyen M, Schachner H, et al. Glomerular Collagen Deposition and Lipocalin-2 Expression Are Early Signs of Renal Injury in Prediabetic Obese Rats. International Journal of Molecular Sciences. 2019; 20(17):4266. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174266
Chicago/Turabian StyleBukosza, Eva Nora, Tamás Kaucsár, Mária Godó, Enikő Lajtár, Pál Tod, Gábor Koncsos, Zoltán V. Varga, Tamás Baranyai, Minh Tu Nguyen, Helga Schachner, and et al. 2019. "Glomerular Collagen Deposition and Lipocalin-2 Expression Are Early Signs of Renal Injury in Prediabetic Obese Rats" International Journal of Molecular Sciences 20, no. 17: 4266. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174266