Transgelin, a p53 and PTEN-Upregulated Gene, Inhibits the Cell Proliferation and Invasion of Human Bladder Carcinoma Cells In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Expressions of TAGLN in Bladder Smooth Muscle Cells, Fibroblast Cells, Normal Epithelial Cells, and Carcinoma Cells
2.2. Expressions of TAGLN in Paired Human Bladder Tissues
2.3. TAGLN’s Localization is Predominantly Cytosolic and with F-actin
2.4. The Effects of TAGLN on Cell Proliferation in Bladder Carcinoma Cells
2.5. The Effects of TAGLN on Cell Invasion in Bladder Carcinoma Cells
2.6. The Effect of the Ectopic Overexpression of TAGLN on the Tumorigenesis of Bladder Carcinoma HT1376 Cells
2.7. p53 and PTEN Upregulated TAGLN Expression in Bladder Carcinoma Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Chemicals
4.2. Tissue Collection and Analysis
4.3. Expression Vector and Stable Transfection
4.4. Knock-Down TAGLN, p53, and PTEN
4.5. Immunoblot Assays
4.6. Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-qPCR)
4.7. Cell Immunofluorescence and F-Actin Staining
4.8. EdU Staining Proliferation Assay
4.9. EdU Flow Cytometry Assay
4.10. Ki67 Proliferation Assay
4.11. Cell Migration Assay
4.12. Matrigel Invasion Assay
4.13. Xenograft Animal Model
4.14. TAGLN Reporter Vector and Reporter Assays
4.15. Statistical Analysis
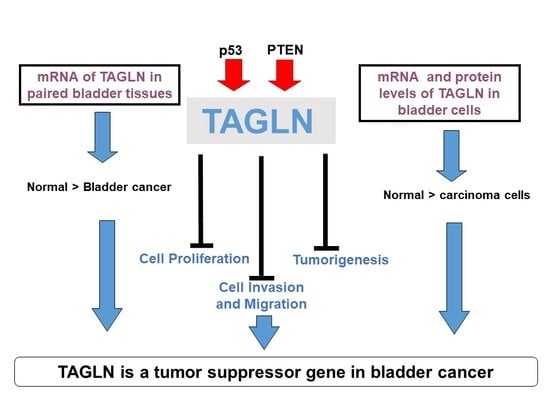
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Camoretti-Mercado, B.; Forsythe, S.M.; LeBeau, M.M.; Espiosa, R.; Vieira, J.E.; Halayko, A.J.; Willadsen, S.; Kurtz, B.; Ober, C.; Evans, G.A.; et al. Expression and cytogenetic localization of the human SM22 gene (TAGLN). Genomics 1998, 49, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yao, J.H.; Cheng, L.; Wei, D.W.; Xue, J.L.; Lu, D.R. Molecular cloning and expression of a smooth muscle-specific gene SM22alpha in zebrafish. Biochem. Biophys. Res. Comm. 2003, 312, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Thompson, O.; Moghraby, J.S.; Ayscough, K.R.; Winder, S.J. Depletion of the actin bundling protein SM22/transgelin increases actin dynamics and enhances the tumourigenic phenotypes of cells. BMC Cell Biol. 2012, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Roehrl, M.H.; Wang, J.Y. Tissue proteomics reveals differential and compartment-specific expression of the homologs transgelin and transgelin-2 in lung adenocarcinoma and its stroma. J. Proteome Res. 2009, 8, 5610–5618. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.M.; Rogers-Graham, K.; Der, C.J. Loss of transgelin in breast and colon tumors and in RIE-1 cells by Ras deregulation of gene expression through Raf-independent pathways. J. Biol. Chem. 2002, 277, 9790–9799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, K.; Zhang, J.; Liu, S.S.; Dai, L.; Zhang, J.Y. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J. Proteome Res. 2011, 10, 2863–2872. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Liang, Y.; Shao, J.; Peng, F.; Sun, M.; Xu, N.; Li, X.; Wang, R.; Liu, S.; et al. A controversial tumor marker: Is SM22 a proper biomarker for gastric cancer cells? J. Proteome Res. 2007, 6, 3304–3312. [Google Scholar] [CrossRef]
- Yu, B.; Chen, X.; Li, J.; Qu, Y.; Su, L.; Peng, Y.; Huang, J.; Yan, J.; Yu, Y.; Gu, Q.; et al. Stromal fibroblasts in the microenvironment of gastric carcinomas promote tumor metastasis via upregulating TAGLN expression. BMC Cell Biol. 2013, 14, 17. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Deng, Y.J.; Wang, S.; Liu, C.; Jin, H.; Ding, Y.Q. Transgelin as a suppressor is associated with poor prognosis in colorectal carcinoma patients. Modern Pathol. 2009, 22, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.L.; Liu, Y.B.; Liu, Y.P.; Du, B.L.; Li, Y.; Han, M.; Li, B.H. Reduced expression of SM22 is correlated with low autophagy activity in human colorectal cancer. Pathol. Res. Prac. 2013, 209, 237–243. [Google Scholar] [CrossRef]
- Chunhua, L.; Donglan, L.; Xiuqiong, F.; Lihua, Z.; Qin, F.; Yawei, L.; Liang, Z.; Ge, W.; Linlin, J.; Ping, Z.; et al. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J. Nutr. Biochem. 2013, 24, 1766–1775. [Google Scholar] [CrossRef]
- Pang, J.; Liu, W.P.; Liu, X.P.; Li, L.Y.; Fang, Y.Q.; Sun, Q.P.; Liu, S.J.; Li, M.T.; Su, Z.L.; Gao, X. Profiling protein markers associated with lymph node metastasis in prostate cancer by DIGE-based proteomics analysis. J. Proteome Res. 2010, 9, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Park, H.J.; Kim, D.K.; Kim, Y.B.; Cheong, J.Y.; Lee, K.J.; Cho, S.W. Loss of SM22 is a characteristic signature of colon carcinogenesis and its restoration suppresses colon tumorigenicity in vivo and in vitro. Cancer 2010, 116, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Kojima, M.; Higuchi, Y.; Nishizawa, Y.; Kobayashi, A.; Ito, M.; Saito, N.; Ochiai, A. Gene expression profile in the activation of subperitoneal fibroblasts reflects prognosis of patients with colon cancer. Int. J. Cancer 2016, 138, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Chen, Q.; Lin, Y. AKT and JNK Signaling pathways increase the metastatic potential of colorectal cancer cells by altering transgelin expression. Dig. Dis. Sci. 2016, 61, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dong, L.; Zhang, R.; Ying, K.; Shen, H. Transgelin overexpression in lung adenocarcinoma is associated with tumor progression. Int. J. Mol. Med. 2014, 34, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Li, L.S.; Kim, H.; Rhee, H.; Kim, S.H.; Shin, D.H.; Chung, K.Y.; Park, K.S.; Paik, Y.K.; Chang, J.; Kim, H. Proteomic analysis distinguishes basaloid carcinoma as a distinct subtype of nonsmall cell lung carcinoma. Proteomics 2004, 4, 3394–3400. [Google Scholar] [CrossRef]
- Chiavegato, A.; Roelofs, M.; Franch, R.; Castellucci, E.; Sarinella, F.; Sartore, S. Differential expression of SM22 isoforms in myofibroblasts and smooth muscle cells from rabbit bladder. J. Muscle Res. Cell Motil. 1999, 20, 133–146. [Google Scholar] [CrossRef]
- Zaravinos, A.; Lambrou, G.I.; Boulalas, I.; Delakas, D.; Spandidos, D.A. Identification of common differentially expressed genes in urinary bladder cancer. PLoS ONE 2011, 6, e18135. [Google Scholar] [CrossRef]
- Chen, R.; Feng, C.; Xu, Y. Cyclin-dependent kinase-associated protein Cks2 is associated with bladder cancer progression. J. Int. Med. Res. 2011, 39, 533–540. [Google Scholar] [CrossRef]
- Antomi, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Goodison, S.; Rosser, C.J.; Urquidi, V. Bladder cancer detection and monitoring: Assessment of urine- and blood based marker tests. Mol. Diag. Ther. 2013, 17, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.M.; Adejoro, O.; Schwartz, I.; Ziegelmann, M.; Elliott, S.; Konety, B.R. The prevalence and impact of urinary marker testing in patients with bladder cancer. J. Urol. 2018, 199, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, S.; Zhan, Y.; He, A.; Fang, D.; Gong, Y.; Li, X.; Zhou, L. TGF-β-induced transgelin promotes bladder cancer metastasis by upregulating epithelial-mesenchymal transition and invadopodia formation. EBioMedicine 2019, 47, 208–220. [Google Scholar] [CrossRef]
- Ning, X.; Deng, Y. Identification of key pathways and genes influencing prognosis in bladder urothelial carcinoma. Onco. Targets Ther. 2017, 10, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Nenutil, R.; Bouchal, P. Transgelins, cytoskeletal proteins implicated in different aspects of cancer development. Expert. Rev. Proteomics 2014, 11, 149–165. [Google Scholar] [CrossRef]
- Yoshino, H.; Chiyomaru, T.; Enokida, H.; Kawakami, K.; Tatarano, S.; Nishiyama, K.; Nohata, N.; Seki, N.; Nakagawa, M. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br. J. Cancer 2011, 104, 808–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Jiang, M.; Liu, Q.; Han, Z.; Zhao, Y.; Ji, S. miR-145-5p inhibits the proliferation and migration of bladder cancer cells by targeting TAGLN2. Oncol. Lett. 2018, 16, 6355–6360. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.P.; Spary, L.K.; Mason, M.D.; Tabi, Z.; Bewis, I.A.; Clayton, A. Prostate stromal cell proteomic analysis discriminates normal from tumour reactive stroma phenotypes. Oncotarget 2016, 7, 20124–20139. [Google Scholar] [CrossRef]
- Prasad, P.D.; Stanton, J.A.; Assinder, S.J. Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res. 2010, 339, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.S.; Ishikawa, A.; Deguchi, S. Transgelin-1 (SM22α) interacts with actin stress fibers and podosomes in smooth muscle cells without using its actin binding site. Biochem. Biophys. Res. Commun. 2018, 505, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Manikandan, M.; Dawud, R.A.; Alajez, N.M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Mahmood, A. Transgelin is a TGFβ-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016, 7, e2321. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, G.; Gander, R.; Lilg, C.; Lepperdinger, G.; Plas, E.; Berger, P. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech. Ageing Dev. 2005, 126, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Fenne, I.S.; Helland, T.; Flageng, M.H.; Dankel, S.N.; Mellgren, G.; Sagen, J.V. Downregulation of steroid receptor coactivator-2 modulates estrogen-responsive genes and stimulates proliferation of mcf-7 breast cancer cells. PLoS ONE 2013, 8, e70096. [Google Scholar] [CrossRef] [PubMed]
- Pashaie, E.; Guzel, E.; Ozgurses, M.E.; Demirel, G.; Aydin, N.; Ozen, M. A meta-analysis: Identification of common mir-145 target gens that have similar behavior in different GEO datasets. PLoS ONE 2016, 11, e0161491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Yang, Z.M.; Zheng, Y.C.; Chen, Z.D. Transgelin induces apoptosis of human prostate LNCaP cells through its interaction with p53. Asian J. Androl. 2010, 12, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, I.; Drobnjak, M.; Fazzari, M.; Ferrara, J.; Scher, H.I.; Cordon-Cardo, C. Inactivation of the p53 pathway in prostate cancer: Impact on tumor progression. Clin. Cancer Res. 1999, 5, 2082–2088. [Google Scholar] [PubMed]
- Tsui, K.H.; Chiang, K.C.; Feng, T.H.; Chang, K.S.; Lin, Y.H.; Juang, H.H. BTG2 is a tumor suppressor gene and upregulated by p53 and PTEN in human bladder carcinoma cells. Cancer Med. 2018, 7, 184–195. [Google Scholar] [CrossRef]
- El-Deiry, W.S. Regulation of p53 downstream genes. Semin. Cancer Biol. 1998, 8, 345–357. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S.K.; Ro, J.Y. Overexpression of DJ-1 and HSP90alpha, and loss of PTEN associated with invasive urothelial carcinoma of urinary bladder: Possible prognostic markers. Oncol. Lett. 2012, 3, 507–512. [Google Scholar] [PubMed]
- Boosani, C.S.; Agrawal, D.K. PTEN modulators: A patent review. Expert. Opin. Ther. Pat. 2013, 23, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; McLeod, H.L. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anti-cancer Drugs 2005, 16, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Hou, C.P.; Chang, K.S.; Lin, Y.H.; Feng, T.H.; Chen, C.C.; Shin, Y.S.; Juang, H.H. Metallothionein 3 is a hypoxia-upregulated oncogene enhancing cell invasion and tumorigenesis in human bladder carcinoma cells. Int. J. Mol. Sci. 2019, 20, 980. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Hsu, S.Y.; Chung, L.C.; Lin, Y.H.; Feng, T.H.; Lee, T.Y.; Chang, P.L.; Juang, H.H. Growth differentiation factor-15: A p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells. Sci. Rep. 2015, 5, 12870. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Chang, Y.L.; Yang, P.S.; Hou, C.P.; Lin, Y.H.; Lee, B.W.; Feng, T.H.; Juang, H.H. The inhibitory effects of capillarisin on cell proliferation and invasion of prostate carcinoma cells. Cell Prolif. 2018, 51, e12419. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.S.; Tsui, K.H.; Lin, Y.H.; Hou, C.P.; Feng, T.H.; Juang, H.H. Migration and invasion enhancer 1 is an NF-κB-inducing gene enhancing the cell proliferation and invasion ability of human prostate carcinoma cells in vitro and in vivo. Cancers 2019, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Lin, Y.H.; Chung, L.C.; Chuang, S.T.; Feng, T.H.; Chiang, K.C.; Chang, P.L.; Yen, C.L.; Juang, H.H. Prostate-derived ets factor represses tumorigenesis and modulates epithelial-to-mesenchymal transition in bladder carcinoma cells. Cancer Lett. 2016, 375, 142–151. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsui, K.-H.; Lin, Y.-H.; Chang, K.-S.; Hou, C.-P.; Chen, P.-J.; Feng, T.-H.; Juang, H.-H. Transgelin, a p53 and PTEN-Upregulated Gene, Inhibits the Cell Proliferation and Invasion of Human Bladder Carcinoma Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 4946. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194946
Tsui K-H, Lin Y-H, Chang K-S, Hou C-P, Chen P-J, Feng T-H, Juang H-H. Transgelin, a p53 and PTEN-Upregulated Gene, Inhibits the Cell Proliferation and Invasion of Human Bladder Carcinoma Cells In Vitro and In Vivo. International Journal of Molecular Sciences. 2019; 20(19):4946. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194946
Chicago/Turabian StyleTsui, Ke-Hung, Yu-Hsiang Lin, Kang-Shuo Chang, Chen-Pang Hou, Pin-Jung Chen, Tsui-Hsia Feng, and Horng-Heng Juang. 2019. "Transgelin, a p53 and PTEN-Upregulated Gene, Inhibits the Cell Proliferation and Invasion of Human Bladder Carcinoma Cells In Vitro and In Vivo" International Journal of Molecular Sciences 20, no. 19: 4946. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194946