Pisidium coreanum Inhibits Multinucleated Osteoclast Formation and Prevents Estrogen-Deficient Osteoporosis
Abstract
:1. Introduction
2. Results
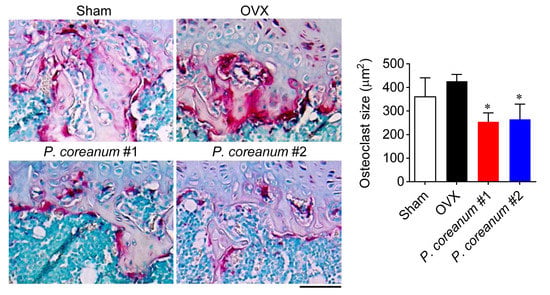
2.1. P. Coreanum Inhibits Osteoclast Differentiation and Prevents Bone Loss in Estrogen-Deficient Mice
2.2. P. Coreanum Inhibits the Fusion during Osteoclast Maturation
3. Discussion
4. Materials and Methods
4.1. Preparation of P. coreanum Powder
4.2. Scanning Electron Microscopy Analysis
4.3. Osteoclast Differentiation
4.4. MTT Assay
4.5. Ovariectomy
4.6. High-Resolution μCT Analysis
4.7. Quantitative Real-Time RT-PCR
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. Camb. Philos. Soc. 2010, 85, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar] [PubMed]
- Mitta, G.; Vandenbulcke, F.; Roch, P. Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Lett. 2000, 486, 185–190. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Li, C.; Ni, D.; Wu, L.; Zhu, L.; Wang, H.; Xu, W. Molecular cloning, expression of a big defensin gene from bay scallop argopecten irradians and the antimicrobial activity of its recombinant protein. Mol. Immunol. 2007, 44, 360–368. [Google Scholar] [CrossRef]
- Marin, F.; Le Roy, N.; Marie, B. The formation and mineralization of mollusk shell. Front. Biosci. (Schol. Ed.) 2012, 4, 1099–1125. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.S. Re-description Pisidium (Neopisidium) coreanum (Veneroida: Sphaeriidae) from Korea. Korean J. Malacol. 2008, 24, 93–96. [Google Scholar]
- Park, J.; Kwon, O.K. Studies on the Development and the Spawning Season of Pisidium (Neopisidium) coreanum (Bivalvia: Sphaeriidae). Korean J. Malacol. 1993, 9, 33–38. [Google Scholar]
- Baek, M.K.; Lee, J.S.; Kang, S.W.; Lee, J.B.; Kang, H.J.; Jo, Y.H.; Noh, M.Y.; Han, Y.S.; Choi, S.H.; Chae, S.H.; et al. Phylogenetic analysis based on metallothionein gene sequence of an indigenous species Pisidium (Neopisidium) coreanum in Korea. Korean J. Malacol. 2009, 25, 153–160. [Google Scholar]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Riggs, B.L.; Melton, L.J.; Robb, R.A.; Camp, J.J.; Atkinson, E.J.; McDaniel, L.; Amin, S.; Rouleau, P.A.; Khosla, S. A population-based assessment of rates of bone loss at multiple skeletal sites: Evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. 2008, 23, 205–214. [Google Scholar] [CrossRef]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in wnt/beta-catenin signaling in mice. J. Orthop. Res. 2017, 35, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. Bone remodeling. Henry Ford Hosp. Med. J. 1988, 36, 143–144. [Google Scholar] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef] [PubMed]
- Jules, J.; Ashley, J.W.; Feng, X. Selective targeting of rank signaling pathways as new therapeutic strategies for osteoporosis. Expert Opin. Ther. Targets 2010, 14, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Wahner, H.W.; Seeman, E.; Offord, K.P.; Dunn, W.L.; Mazess, R.B.; Johnson, K.A.; Melton, L.J., 3rd. Changes in bone mineral density of the proximal femur and spine with aging. Differences between the postmenopausal and senile osteoporosis syndromes. J. Clin. Investig. 1982, 70, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916. [Google Scholar] [CrossRef]
- Reid, I.R. Efficacy, effectiveness and side effects of medications used to prevent fractures. J. Intern. Med. 2015, 277, 690–706. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor nfatc1 (nfat2) integrate rankl signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Nakamura, I.; Duong, L.T.; Rodan, S.B.; Rodan, G.A. Involvement of alpha(v)beta3 integrins in osteoclast function. J. Bone Miner. Metab. 2007, 25, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Fukase, M.; Miyamoto, H.; Matsumoto, T.; Ohue, T. Increase of bone mineral density by calcium supplement with oyster shell electrolysate. Bone Miner. 1990, 11, 85–91. [Google Scholar] [CrossRef]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Qian, Z.J.; Jung, W.K. Beneficial effect of abalone intestine gastro-intestinal digests on osteoblastic MG-63 cell differentiation. J. Aquat. Food Prod. Technol. 2014, 23, 436–446. [Google Scholar] [CrossRef]
- Jeong, J.E.; Kang, S.W.; Hwang, H.J.; Park, S.Y.; Patnaik, B.B.; Kim, C.; Kim, S.; Lee, J.B.; Wang, T.H.; Park, E.B.; et al. Expressed Sequence Tags from the Threatened Freshwater Sphaeriid Clam Species, Pisidium (Neopisidium) coreanum: Discovery of Functional Genomic Resources. Korean Soc. Biotechnol. Bioeng. 2015, 10, 407. [Google Scholar]
- Bar-Shavit, Z. The osteoclast: A multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J. Cell Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef]
- Lees, R.L.; Sabharwal, V.K.; Heersche, J.N. Resorptive state and cell size influence intracellular ph regulation in rabbit osteoclasts cultured on collagen-hydroxyapatite films. Bone 2001, 28, 187–194. [Google Scholar] [CrossRef]
- Hu, Y.; Ek-Rylander, B.; Karlstrom, E.; Wendel, M.; Andersson, G. Osteoclast size heterogeneity in rat long bones is associated with differences in adhesive ligand specificity. Exp. Cell Res. 2008, 314, 638–650. [Google Scholar] [CrossRef]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. Dc-stamp is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp Med. 2005, 202, 345–351. [Google Scholar] [CrossRef]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. V-atpase v0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef]
- Yang, M.; Birnbaum, M.J.; MacKay, C.A.; Mason-Savas, A.; Thompson, B.; Odgren, P.R. Osteoclast stimulatory transmembrane protein (oc-stamp), a novel protein induced by rankl that promotes osteoclast differentiation. J. Cell Physiol. 2008, 215, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Saginario, C.; Sterling, H.; Beckers, C.; Kobayashi, R.; Solimena, M.; Ullu, E.; Vignery, A. Mfr, a putative receptor mediating the fusion of macrophages. Mol. Cell Biol. 1998, 18, 6213–6223. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.J.; Thomas, K.; Huang, C.S.; Gutknecht, M.F.; Botchwey, E.A.; Bouton, A.H. Regulation of osteoclast structure and function by fak family kinases. J. Leukoc. Biol. 2012, 92, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- McDougall, C.; Degnan, B.M. The evolution of mollusc shells. Wiley Interdiscip. Rev. Dev. Biol 2018, 7, e313. [Google Scholar] [CrossRef]
- Gerhard, E.M.; Wang, W.; Li, C.; Guo, J.; Ozbolat, I.T.; Rahn, K.M.; Armstrong, A.D.; Xia, J.; Qian, G.; Yang, J. Design strategies and applications of nacre-based biomaterials. Acta Biomater. 2017, 54, 21–34. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Ko, C.Y.; Kim, H.S.; Shin, H.I.; Kim, T.; Lee, S.H.; Jeong, D. The role of nacreous factors in preventing osteoporotic bone loss through both osteoblast activation and osteoclast inactivation. Biomaterials 2012, 33, 7489–7496. [Google Scholar] [CrossRef]
- Duplat, D.; Gallet, M.; Berland, S.; Marie, A.; Dubost, L.; Rousseau, M.; Kamel, S.; Milet, C.; Brazier, M.; Lopez, E.; et al. The effect of molecules in mother-of-pearl on the decrease in bone resorption through the inhibition of osteoclast cathepsin k. Biomaterials 2007, 28, 4769–4778. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, J.; Zhang, H.; Zhao, F. In vitro osteogenetic activity of pearl. Biomaterials 2006, 27, 281–287. [Google Scholar] [CrossRef]
- Rousseau, M.; Pereira-Mouries, L.; Almeida, M.J.; Milet, C.; Lopez, E. The water-soluble matrix fraction from the nacre of pinctada maxima produces earlier mineralization of mc3t3-e1 mouse pre-osteoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 1–7. [Google Scholar] [CrossRef]
- Westbroek, P.; Marin, F. A marriage of bone and nacre. Nature 1998, 392, 861–862. [Google Scholar] [CrossRef]
- Berland, S.; Delattre, O.; Borzeix, S.; Catonne, Y.; Lopez, E. Nacre/bone interface changes in durable nacre endosseous implants in sheep. Biomaterials 2005, 26, 2767–2773. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.H.; Lee, K.; Kim, M.Y.; Shin, H.-I.; Jeong, D. Pisidium coreanum Inhibits Multinucleated Osteoclast Formation and Prevents Estrogen-Deficient Osteoporosis. Int. J. Mol. Sci. 2019, 20, 6076. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20236076
Choi MH, Lee K, Kim MY, Shin H-I, Jeong D. Pisidium coreanum Inhibits Multinucleated Osteoclast Formation and Prevents Estrogen-Deficient Osteoporosis. International Journal of Molecular Sciences. 2019; 20(23):6076. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20236076
Chicago/Turabian StyleChoi, Mun Hwan, Kyunghee Lee, Mi Yeong Kim, Hong-In Shin, and Daewon Jeong. 2019. "Pisidium coreanum Inhibits Multinucleated Osteoclast Formation and Prevents Estrogen-Deficient Osteoporosis" International Journal of Molecular Sciences 20, no. 23: 6076. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20236076