Serum Biomarkers of Cardiovascular Remodelling Reflect Extra-Valvular Cardiac Damage in Patients with Severe Aortic Stenosis
Abstract
:1. Introduction
Aim of the Study
2. Results
2.1. Characterization of the Study Population
2.2. Comparative Analysis of Clinical Characteristics between the Different Staging Groups and Disease Entities
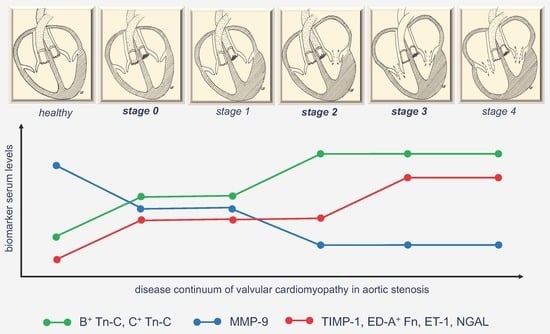
2.3. Analysis of Biomarkers of Cardiovascular Remodelling in Comparison Between Controls and the Different Staging Groups
2.4. Parameters Predicting an Advanced Stage of Extravalvular Cardiac Damage in the Study Population
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Stages of Extra-Valvular Cardiac Damage According to the 2017 Staging Classification
4.3. Blood Samples
4.4. Routine Laboratory Parameters and Quantification of Serum Biomarker Levels
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6 minutes’ walk test |
| Afib | atrial fibrillation |
| B+ Tn-C | B domain containing tenascin-C |
| BNP | brain natriuretic peptide |
| C+ Tn-C | C domain containing tenascin-C |
| CAD | coronary artery disease |
| COPD | chronic obstructive pulmonary disease |
| ECM ED-A+ Fn | extracellular matrix ED-A domain containing fibronectin |
| ET-1 | endothelin 1 |
| GFR | glomerular filtration rate |
| HGAS | high gradient aortic stenosis |
| IVSd | interventricular septum thickness in diastole |
| LGAS | low gradient aortic stenosis |
| LVEF | left ventricular ejection fraction |
| MMP-9 | matrix metalloproteinase 9 |
| NGAL | neutrophil gelatinase associated lipocalin |
| NYHA | New York Heart Association |
| PAD | peripheral artery disease |
| PLFLGAS | paradoxical low flow low gradient aortic stenosis |
| RV | right ventricle |
| SD | standard deviation |
| sPAP | systolic pulmonary artery pressure |
| SR | sinus rhythm |
| STS | Society of Thoracic Surgeons |
| TAVI | transcatheter aortic valve implantation |
| TIMP-1 | tissue inhibitor of metalloproteinase 1 |
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; ESC Scientific Document Group; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.; Thaden, J.J.; Nkomo, V.T. Management of Patients with Aortic Valve Stenosis. Mayo. Clin. Proc. 2018, 93, 488–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podlesnikar, T.; Delgado, V.; Bax, J.J. Imaging of Valvular Heart Disease in Heart Failure. Card. Fail. Rev. 2018, 4, 78–86. [Google Scholar] [CrossRef]
- Annabi, M.S.; Clisson, M.; Clavel, M.A.; Pibarot, P. Workup and Management of Patients with Paradoxical Low-Flow, Low-Gradient Aortic Stenosis. Curr. Treat. Opt. Cardiovasc. Med. 2018, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Annabi, M.S.; Touboul, E.; Dahou, A.; Burwash, I.G.; Bergler-Klein, J.; Enriquez-Sarano, M.; Orwat, S.; Baumgartner, H.; Mascherbauer, J.; Mundigler, G.; et al. Dobutamine Stress Echocardiography for Management of Low-Flow, Low-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 475–485. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Pibarot, P.; Dumesnil, J.G. Improving assessment of aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Lauten, A.; Figulla, H.R.; Mollmann, H.; Holzhey, D.; Kotting, J.; Beckmann, A.; Veit, C.; Cremer, J.; Kuck, K.H.; Lange, R.; et al. TAVI for low-flow, low-gradient severe aortic stenosis with preserved or reduced ejection fraction: A subgroup analysis from the German Aortic Valve Registry (GARY). EuroIntervention 2014, 10, 850–859. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Ielasi, A.; Latib, A.; Tespili, M.; Donatelli, F. Current results and remaining challenges of trans-catheter aortic valve replacement expansion in intermediate and low risk patients. Int. J. Cardiol. Heart Vasc. 2019, 23, 100375. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.Q.; Xiao, Y.; Yuan, Y.; Ma, Z.G.; Liao, H.H.; Liu, C.; Zhu, J.X.; Yang, Z.; Deng, W.; Tang, Q.Z. Mechanisms contributing to cardiac remodelling. Clin. Sci. 2017, 131, 2319–2345. [Google Scholar] [CrossRef]
- MacLean, J.; Pasumarthi, K.B. Signaling mechanisms regulating fibroblast activation, phenoconversion and fibrosis in the heart. Ind. J. Biochem. Biophys. 2014, 51, 476–482. [Google Scholar]
- Abraham, D.; Dashwood, M. Endothelin—Role in vascular disease. Rheumatology 2008, 47 (Suppl 5), v23–v24. [Google Scholar] [CrossRef] [Green Version]
- Suthahar, N.; Meijers, W.C.; Sillje, H.H.W.; de Boer, R.A. From Inflammation to Fibrosis-Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr. Heart Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivalingam, Z.; Erik Magnusson, N.; Grove, E.L.; Hvas, A.M.; Dalby Kristensen, S.; Bojet Larsen, S. Neutrophil gelatinase-associated lipocalin (NGAL) and cardiovascular events in patients with stable coronary artery disease. Scand. J. Clin. Lab. Investig. 2018, 78, 470–476. [Google Scholar] [CrossRef]
- Bowers, S.L.; Banerjee, I.; Baudino, T.A. The extracellular matrix: At the center of it all. J. Mol. Cell Cardiol. 2010, 48, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Spinale, F.G. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef]
- Franz, M.; Berndt, A.; Altendorf-Hofmann, A.; Fiedler, N.; Richter, P.; Schumm, J.; Fritzenwanger, M.; Figulla, H.R.; Brehm, B.R. Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy. Eur. J. Heart Fail. 2009, 11, 1057–1062. [Google Scholar] [CrossRef]
- Franz, M.; Brehm, B.R.; Richter, P.; Gruen, K.; Neri, D.; Kosmehl, H.; Hekmat, K.; Renner, A.; Gummert, J.; Figulla, H.R.; et al. Changes in extra cellular matrix remodelling and re-expression of fibronectin and tenascin-C splicing variants in human myocardial tissue of the right atrial auricle: Implications for a targeted therapy of cardiovascular diseases using human SIP format antibodies. J. Mol. Histol. 2010, 41, 39–50. [Google Scholar]
- Ziffels, B.; Ospel, J.; Grun, K.; Neri, D.; Pfeil, A.; Fritzenwanger, M.; Figulla, H.R.; Jung, C.; Berndt, A.; Franz, M. Detection of Soluble ED-A(+) Fibronectin and Evaluation as Novel Serum Biomarker for Cardiac Tissue Remodeling. Dis. Mark. 2016, 2016, 3695454. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.; Jung, C.; Lauten, A.; Figulla, H.R.; Berndt, A. Tenascin-C in cardiovascular remodeling: Potential impact for diagnosis, prognosis estimation and targeted therapy. Cell Adh. Migr. 2015, 9, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Rohm, I.; Grun, K.; Muller, L.M.; Kretzschmar, D.; Fritzenwanger, M.; Yilmaz, A.; Lauten, A.; Jung, C.; Schulze, P.C.; Berndt, A.; et al. Increased Serum Levels of Fetal Tenascin-C Variants in Patients with Pulmonary Hypertension: Novel Biomarkers Reflecting Vascular Remodeling and Right Ventricular Dysfunction? Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, M.; Doll, F.; Grun, K.; Richter, P.; Kose, N.; Ziffels, B.; Schubert, H.; Figulla, H.R.; Jung, C.; Gummert, J.; et al. Targeted delivery of interleukin-10 to chronic cardiac allograft rejection using a human antibody specific to the extra domain A of fibronectin. Int. J. Cardiol. 2015, 195, 311–322. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanos, N.K.; Theocharis, A.D.; Neill, T.; Iozzo, R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019, 75–76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Berndt, A.; Neri, D.; Galler, K.; Grun, K.; Porrmann, C.; Reinbothe, F.; Mall, G.; Schlattmann, P.; Renner, A.; et al. Matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, B(+) tenascin-C and ED-A(+) fibronectin in dilated cardiomyopathy: Potential impact on disease progression and patients’ prognosis. Int. J. Cardiol. 2013, 168, 5344–5351. [Google Scholar] [CrossRef]
- Marchesi, C.; Dentali, F.; Nicolini, E.; Maresca, A.M.; Tayebjee, M.H.; Franz, M.; Guasti, L.; Venco, A.; Schiffrin, E.L.; Lip, G.Y.; et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: A systematic review and meta-analysis. J. Hypertens. 2012, 30, 3–16. [Google Scholar] [CrossRef]
- de Torres, J.P.; Casanova, C.; Pinto-Plata, V.; Varo, N.; Restituto, P.; Cordoba-Lanus, E.; Baz-Davila, R.; Aguirre-Jaime, A.; Celli, B.R. Gender differences in plasma biomarker levels in a cohort of COPD patients: A pilot study. PLoS ONE 2011, 6, e16021. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, I.S.; Janson, C.; Lind, L.; Hulthe, J.; Gunnbjornsdottir, M.; Sundstrom, J. Serum levels of matrix metalloproteinase-9, tissue inhibitors of metalloproteinase-1 and their ratio are associated with impaired lung function in the elderly: A population-based study. Respirology 2010, 15, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Matusiak-Bruckner, M.; Richter, P.; Grun, K.; Ziffels, B.; Neri, D.; Maschek, H.; Schulz, U.; Pfeil, A.; Jung, C.; et al. De novo expression of fetal ED-A(+) fibronectin and B (+) tenascin-C splicing variants in human cardiac allografts: Potential impact for targeted therapy of rejection. J. Mol. Histol. 2014, 45, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Berndt, A.; Grun, K.; Kuethe, F.; Fritzenwanger, M.; Figulla, H.R.; Jung, C. Serum levels of tenascin-C variants in congestive heart failure patients: Comparative analysis of ischemic, dilated, and hypertensive cardiomyopathy. Clin. Lab. 2014, 60, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Z.; Peng, Y.; Gao, H.; Wang, Y.; Zhao, J.; Pan, C. Plasma neutrophil gelatinase-associated lipocalin levels are associated with the presence and severity of coronary heart disease. PLoS ONE 2019, 14, e0220841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.C.; Potoka, K.C.; Champion, H.C.; Mora, A.L.; Gladwin, M.T. Pulmonary arterial hypertension: The clinical syndrome. Circ. Res. 2014, 115, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Hartopo, A.B.; Arfian, N.; Nakayama, K.; Suzuki, Y.; Yagi, K.; Emoto, N. Endothelial-derived endothelin-1 promotes pulmonary vascular remodeling in bleomycin-induced pulmonary fibrosis. Physiol Res. 2018, 67 (Suppl 1), S185–S197. [Google Scholar] [CrossRef]
- Parikh, R.V.; Khush, K.; Pargaonkar, V.S.; Luikart, H.; Grimm, D.; Yu, M.; Okada, K.; Honda, Y.; Yeung, A.C.; Valantine, H.; et al. Association of Endothelin-1 With Accelerated Cardiac Allograft Vasculopathy and Late Mortality Following Heart Transplantation. J. Card. Fail. 2019, 25, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, I.; Pugliese, N.R.; Calogero, E.; Conte, L.; Mazzanti, M.C.; Scatena, C.; Scopelliti, C.; Tantillo, E.; Passiatore, M.; Angelillis, M.; et al. MicroRNAs distribution in different phenotypes of Aortic Stenosis. Sci. Rep. 2018, 8, 9953. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.; Grun, K.; Betge, S.; Rohm, I.; Ndongson-Dongmo, B.; Bauer, R.; Schulze, P.C.; Lichtenauer, M.; Petersen, I.; Neri, D.; et al. Lung tissue remodelling in MCT-induced pulmonary hypertension: A proposal for a novel scoring system and changes in extracellular matrix and fibrosis associated gene expression. Oncotarget 2016, 7, 81241–81254. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.; Neri, D.; Berndt, A. Chronic cardiac allograft rejection: Critical role of ED-A(+) fibronectin and implications for targeted therapy strategies. J. Pathol. 2012, 226, 557–561. [Google Scholar] [CrossRef]
- Franz, M.; Berndt, A.; Grun, K.; Richter, P.; Kosmehl, H.; Neri, D.; Gummert, J.; Figulla, H.R.; Brehm, B.R.; Renner, A. Expression of extra domain A containing fibronectin in chronic cardiac allograft rejection. J. Heart Lung Transplant. 2011, 30, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gellen, B.; Thorin-Trescases, N.; Thorin, E.; Gand, E.; Sosner, P.; Brishoual, S.; Rigalleau, V.; Montaigne, D.; Javaugue, V.; Pucheu, Y.; et al. Serum tenascin-C is independently associated with increased major adverse cardiovascular events and death in individuals with type 2 diabetes: A French prospective cohort. Diabetologia 2020, 63, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Blazhev, A.; Atanasova, M.; Dimitrova, A. Serum Concentrations of Endothelin-1 and Matrix Metalloproteinases-2, -9 in Pre-Hypertensive and Hypertensive Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaider, A.; Kozakowski, N.; Weninger, W.J.; Nanobachvili, J.; Wojta, J.; Huk, I.; Demyanets, S.; et al. Neutrophil gelatinase associated lipocalin (NGAL) is elevated in type 2 diabetics with carotid artery stenosis and reduced under metformin treatment. Cardiovasc. Diabetol. 2017, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, M.; Munoz-Esquerre, M.; Huertas, D.; Montes, A.; Molina-Molina, M.; Manresa, F.; Dorca, J.; Santos, S. Inflammatory markers and circulating extracellular matrix proteins in patients with chronic obstructive pulmonary disease and left ventricular diastolic dysfunction. Clin. Respir. J. 2017, 11, 859–866. [Google Scholar] [CrossRef]
- Man, S.F.; Xing, L.; Connett, J.E.; Anthonisen, N.R.; Wise, R.A.; Tashkin, D.P.; Zhang, X.; Vessey, R.; Walker, T.G.; Celli, B.R.; et al. Circulating fibronectin to C-reactive protein ratio and mortality: A biomarker in COPD? Eur. Respir. J. 2008, 32, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Spiropoulos, K.; Trakada, G.; Nikolaou, E.; Prodromakis, E.; Efremidis, G.; Pouli, A.; Koniavitou, A. Endothelin-1 levels in the pathophysiology of chronic obstructive pulmonary disease and bronchial asthma. Respir. Med. 2003, 97, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.S.; Kwon, S.O.; Kim, J.; Kim, W.J. Neutrophil gelatinase-associated lipocalin as a complementary biomarker for the asthma-chronic obstructive pulmonary disease overlap. J. Thorac. Dis. 2018, 10, 5047–5056. [Google Scholar] [CrossRef]
- Ferrari, G.; Sainger, R.; Beckmann, E.; Keller, G.; Yu, P.J.; Monti, M.C.; Galloway, A.C.; Weiss, R.L.; Vernick, W.; Grau, J.B. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J. Surg. Res. 2010, 163, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cagirci, G.; Cay, S.; Canga, A.; Karakurt, O.; Yazihan, N.; Kilic, H.; Topaloglu, S.; Aras, D.; Demir, A.D.; Akdemir, R. Association between plasma asymmetrical dimethylarginine activity and severity of aortic valve stenosis. J. Cardiovasc. Med. 2011, 12, 96–101. [Google Scholar] [CrossRef]
- Kruger, R.; Rasmussen, L.M.; Argraves, W.S.; Eugen-Olsen, J.; Nielsen, O.W.; Blyme, A.; Willenheimer, R.; Wachtell, K.; Olsen, M.H. Extracellular matrix biomarker, fibulin-1, is closely related to NT-proBNP and soluble urokinase plasminogen activator receptor in patients with aortic valve stenosis (the SEAS study). PLoS ONE 2014, 9, e101522. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.W.; Bang, C.N.; Eugen-Olsen, J.; Olsen, M.H.; Boman, K.; Ray, S.; Gohlke-Barwolf, C.; Kesaniemi, Y.A.; Jeppesen, J.L.; Wachtell, K. SuPAR Predicts Cardiovascular Events and Mortality in Patients With Asymptomatic Aortic Stenosis. Can. J. Cardiol. 2016, 32, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Tastet, L.; Bergler-Klein, J.; Pibarot, P.; Clavel, M.A. Blood, tissue and imaging biomarkers in calcific aortic valve stenosis: Past, present and future. Curr. Opin. Cardiol. 2018, 33, 125–133. [Google Scholar] [CrossRef] [PubMed]





| Parameter | TAVI Collective (n = 94) | Controls (n = 37) | p-value |
|---|---|---|---|
| Age (mean ± SD, years) | 78.3 ± 7.3 | 66.2 ± 6.5 | <0.001 |
| Male (%) | 46.8 | 35.1 | 0.227 |
| Society of Thoracic Surgeons (STS)-Score (mean ± SD) | 4.4 ± 2.8 | N/A | |
| New York Heart Association (NYHA) I (%) | 7.4 | N/A | |
| NYHA II (%) | 29.8 | N/A | |
| NYHA III (%) | 58.5 | N/A | |
| NYHA IV (%) | 4.3 | N/A | |
| NYHA ≤ II (%) | 37.2 | N/A | |
| NYHA > II (%) | 62.8 | N/A | |
| Angina pectoris (%) | 31.9 | 71.4 (n = 35) | <0.001 |
| Coronary Artery Disease (CAD) (%) | 65.9 | 0 | <0.001 |
| Peripheral Artery Disease (PAD) (%) | 13.8 | N/A | |
| Diabetes (%) | 44.7 | 18.9 | 0.008 |
| Chronic Obstructive Pulmonary Disease (COPD) (%) | 24.5 | 2.7 | 0.004 |
| Atrial fibrillation (%) | 41.5 | 18.9 | 0.015 |
| 6-minutes’ walk test (6MWT) (mean ± SD, meters) | 168 ± 133 | N/A | |
| Glomerular filtration rate (GFR) ≤ 30 mL/min (%) | 13.8 | 0 | 0.018 |
| Dialysis (%) | 5.3 | 0 | 0.154 |
| Brain natriuretic peptide (BNP) ≥ 100 pg/mL (%) | 91 | 5.6 (n = 36) | <0.001 |
| Left ventricular ejection fraction (LVEF) (%) | 55.7 ± 13.8 | 67.8 ± 6.9 | <0.001 |
| Interventricular septal thickness at end-diastole (IVSd) ≥ 12 mm (%) | 92 (n = 87) | 52.2 (n = 23) | <0.001 |
| Mitral regurgitation ≥ II° (%) | 26.6 | 2.7 | 0.002 |
| Tricuspid regurgitation ≥ II° (%) | 18.1 | 2.7 | 0.022 |
| Right ventricular (RV) Dysfunction (%) | 9.6 | 0 | 0.052 |
| Systolic pulmonary artery pressure (sPAP) ≥ 35 mmHg (%) | 77.6 (n = 58) | 0 (n = 36) | <0.001 |
| Stage 0–4 (%): | |||
| 0 | 4.3 | N/A | |
| 1 | 16 | N/A | |
| 2 | 54.3 | N/A | |
| 3 | 16 | N/A | |
| 4 | 9.6 | N/A | |
| Aortic stenosis (AS) entity (%) | |||
| High-gradient aortic stenosis (HGAS) | 72.3 | N/A | |
| Low gradient aortic stenosis (LGAS) | 17 | N/A | |
| Paradoxical low flow LGAS (PLFLGAS) | 10.6 | N/A | |
| Type of TAVI Prosthesis | |||
| Edwards SAPIEN 3, ballon-expandable (%) | 47.9 | N/A | |
| CoreValveTMEvolutTM R, self-expandable (%) | 44.7 | N/A | |
| ACURATE neoTM, self-expandable (%) | 7.4 | N/A | |
| Parameter | Staging Group 1 (n = 19) | Staging Group 2 (n = 51) | Staging Group 3 (n = 24) | p-value Group 1 vs. 2 | p-value Group 1 vs. 3 | p-value Group 2 vs. 3 |
|---|---|---|---|---|---|---|
| Age (mean ± SD, years) | 76.5 ± 6.9 | 79 ± 7.7 | 78.2 ± 6.9 | 0.202 | 0.641 | 0.422 |
| Male (%) | 42.2 | 49 | 45.8 | 0.609 | 0.809 | 0.798 |
| STS score (mean ± SD) | 2.9 ± 1.7 | 4.4 ± 2.5 | 5.4 ± 2.7 | 0.016 | 0.001 | 0.084 |
| NYHA I (%) | 0 | 9.8 | 8.3 | 0.160 | 0.203 | 0.839 |
| NYHA II (%) | 31.6 | 33.3 | 20.8 | 0.890 | 0.428 | 0.271 |
| NYHA III (%) | 63.2 | 52.9 | 66.7 | 0.447 | 0.813 | 0.265 |
| NYHA IV (%) | 5.3 | 3.9 | 4.2 | 0.807 | 0.867 | 0.960 |
| NYHA ≤ II (%) | 31.6 | 43.1 | 29.2 | 0.383 | 0.866 | 0.250 |
| NYHA > II (%) | 68.4 | 56.9 | 70.8 | 0.383 | 0.866 | 0.250 |
| Angina pectoris (%) | 42.1 | 35.3 | 16.7 | 0.603 | 0.068 | 0.101 |
| CAD (%) | 57.9 | 62.7 | 79.2 | 0.713 | 0.136 | 0.158 |
| PAD (%) | 10.5 | 15.7 | 12.5 | 0.586 | 0.843 | 0.718 |
| Diabetes (%) | 31.6 | 54.9 | 33.3 | 0.106 | 0.904 | 0.105 |
| COPD (%) | 31.6 | 27.5 | 12.5 | 0.736 | 0.131 | 0.152 |
| Atrial fibrillation (%) | 0 | 45.1 | 66.7 | <0.001 | <0.001 | 0.083 |
| 6MWT (mean ± SD, meters) | 260 ± 140 | 141 ± 132 | 107 ± 81 | 0.014 | 0.036 | 0.558 |
| GFR ≤ 30 mL/min (%) | 5.3 | 9.8 | 29.2 | 0.549 | 0.048 | 0.034 |
| Dialysis (%) | 0 | 3.9 | 12.5 | 0.385 | 0.114 | 0.168 |
| BNP ≥ 100 pg/mL (%) | 68.4 (n = 18) | 93.9 (n = 49) | 100 (n = 22) | 0.016 | 0.009 | 0.239 |
| LVEF (%) | 60.4 ± 11.3 | 56.7 ± 12.1 | 49.7 ± 17.1 | 0.272 | 0.057 | 0.130 |
| IVSd≥ 12 mm (%) | 94.4 (n = 18) | 95.9 (n = 49) | 80 (n = 20) | 0.797 | 0.194 | 0.035 |
| Mitral regurgitation ≥ II° (%) | 0 | 31.4 | 37.5 | 0.006 | 0.003 | 0.602 |
| Tricuspid regurgitation ≥ II° (%) | 0 | 0 | 70.8 | 1.0 | <0.001 | <0.001 |
| RV Dysfunction (%) | 0 | 0 | 37.5 | 1.0 | 0.003 | <0.001 |
| sPAP≥ 35 mmHg (%) | 50 (n = 6) | 73.3 (n = 30) | 90.9 (n = 22) | 0.264 | 0.023 | 0.116 |
| AS entity (%) | ||||||
| HGAS | 84.2 | 82.4 | 41.7 | 0.856 | 0.005 | <0.001 |
| LGA) | 5.3 | 11.8 | 37.5 | 0.423 | 0.014 | 0.010 |
| PLFLGAS | 10.5 | 5.9 | 20.8 | 0.505 | 0.369 | 0.052 |
| Edwards SAPIEN 3, ballon-expandable (%) | 47.4 | 51 | 41.7 | 0.790 | 0.712 | 0.454 |
| CoreValveTMEvolutTM R, self-expandable (%) | 47.4 | 41.2 | 50 | 0.644 | 0.865 | 0.476 |
| ACURATE neoTM, self-expandable (%) | 5.3 | 7.8 | 8.3 | 0.711 | 0.698 | 0.942 |
| Biomarker | Controls (n = 37) | AS-Patients (n = 94) | p-value |
|---|---|---|---|
| MMP-9 (ng/mL, median ± SD) | 602.05 ± 305.17 | 399.95 ± 323.6 | 0.003 |
| TIMP-1 (ng/mL, median ± SD) | 159.1 ± 42.66 | 177.75 ± 88.68 | 0.018 |
| B+ Tn-C (ng/mL, median ± SD) | 362.11 ± 380.05 | 794.05 ± 775.53 | <0.001 |
| C+ Tn-C (ng/mL, median ± SD) | 66.08 ± 27.37 | 70.03 ± 51.96 | 0.366 |
| ED-A+ Fn (μg/mL, median ± SD) | 8.82 ± 10.22 | 13.31 ± 12.08 | 0.116 |
| ED-B+ Fn (μg/mL, median ± SD) | 3.06 ± 2.10 | 5.25 ± 3.23 | 0.012 |
| ET-1 (ng/mL, median ± SD) | 1.35 ± 0.38 | 2.49 ± 1.19 | <0.001 |
| NGAL (ng/mL, median ± SD) | 83.14 ± 32.98 | 142.64 ± 146.42 | <0.001 |
| Biomarker | Staging Group 1 (n = 19) | Staging Group 2 (n = 51) | Staging Group 3 (n = 24) | p-value Group 1 vs. 2 | p-value Group 1 vs. 3 | p-value Group 2 vs. 3 |
|---|---|---|---|---|---|---|
| MMP-9 (ng/mL, median ± SD) | 528.11 ± 418.53 | 353.60 ± 288.08 | 411.94 ± 292.48 | 0.038 | 0.271 | 0.413 |
| TIMP-1 (ng/mL, median ± SD) | 172.19 ± 53.99 | 174.32 ± 80.74 | 230.44 ± 112.44 | 0.853 | 0.056 | 0.046 |
| B+ Tn-C (ng/mL, median ± SD) | 571.19 ± 483.34 | 1081.68 ± 788.11 | 720.93 ± 841.37 | 0.002 | 0.065 | 0.343 |
| C+ Tn-C (ng/mL, median ± SD) | 45.88 ± 26.12 | 73.77 ± 52.69 | 76.53 ± 59.31 | 0.007 | 0.004 | 0.729 |
| ED-A+ Fn (μg/mL, median ± SD) | 10.30 ± 5.95 | 10.91 ± 11.90 | 20.65 ± 14.19 | 0.838 | 0.026 | 0.018 |
| ED-B+ Fn (μg/mL, median ± SD) | 5.05 ± 2.73 | 5.16 ± 3.50 | 6.22 ± 3.07 | 0.890 | 0.353 | 0.367 |
| ET-1 (ng/mL, median ± SD) | 2.20 ± 1.24 | 2.47 ± 1.06 | 3.40 ± 1.28 | 0.146 | 0.010 | 0.010 |
| NGAL (ng/mL, median ± SD) | 122.84 ± 67.24 | 136.41 ± 159.12 | 181.57 ± 158.85 | 0.561 | 0.012 | 0.037 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bäz, L.; Dannberg, G.; Grün, K.; Westphal, J.; Möbius-Winkler, S.; Jung, C.; Pfeil, A.; Schulze, P.C.; Franz, M. Serum Biomarkers of Cardiovascular Remodelling Reflect Extra-Valvular Cardiac Damage in Patients with Severe Aortic Stenosis. Int. J. Mol. Sci. 2020, 21, 4174. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114174
Bäz L, Dannberg G, Grün K, Westphal J, Möbius-Winkler S, Jung C, Pfeil A, Schulze PC, Franz M. Serum Biomarkers of Cardiovascular Remodelling Reflect Extra-Valvular Cardiac Damage in Patients with Severe Aortic Stenosis. International Journal of Molecular Sciences. 2020; 21(11):4174. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114174
Chicago/Turabian StyleBäz, Laura, Gudrun Dannberg, Katja Grün, Julian Westphal, Sven Möbius-Winkler, Christian Jung, Alexander Pfeil, P. Christian Schulze, and Marcus Franz. 2020. "Serum Biomarkers of Cardiovascular Remodelling Reflect Extra-Valvular Cardiac Damage in Patients with Severe Aortic Stenosis" International Journal of Molecular Sciences 21, no. 11: 4174. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114174