The Heterooligomerization of Human Small Heat Shock Proteins Is Controlled by Conserved Motif Located in the N-Terminal Domain
Abstract
:1. Introduction
2. Results
2.1. Characterization of Analyzed Proteins
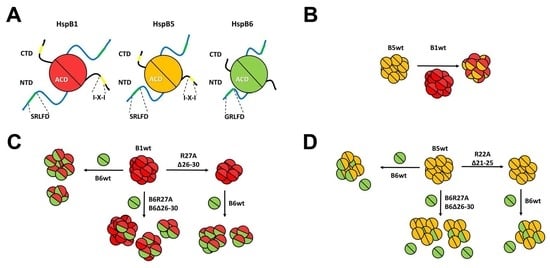
2.2. Effect of R/A and Δ Mutations on the Interaction of HspB1 and HspB6
2.3. Effect of R/A and Δ Mutations on the Interaction of HspB5 and HspB6
2.4. Lack of Interaction of HspB8 and Its Mutants with HspB1 and HspB5
2.5. Formation of Heterooligomeric Complexes between HspB1 and HspB5
3. Discussion
4. Material and Methods
4.1. Cloning of the Wild-Type Proteins and Their Mutants
4.2. Expression and Purification of Recombinant Proteins
4.3. Formation of Heterooligomeric Complexes
4.4. Analysis of Heterooligomeric Complexes
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DTT | dithiothreitol |
| ME | mercaptoethanol |
| PMSF | phenylmethanesulphonyl fluoride |
| SDS | sodium dodecyl sulfate |
| SEC | size-exclusion chromatography |
| TCA | trichloroacetic acid |
| WT | wild type |
References
- Haslbeck, M.; Vierling, E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowska, M.K.; Baughman, H.E.R.; Woods, C.N.; Klevit, R.E. Mechanisms of small heat shock proteins. Cold Spring Harb. Perspect. Biol. 2019, 11, a034025. [Google Scholar] [CrossRef] [PubMed]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta 2015, 1854, 291–319. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, J.M.; Rest, J.S.; Welsh, M.J.; Benndorf, R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones 2003, 8, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Kappe, G.; Franck, E.; Verschuure, P.; Boelens, W.C.; Leunissen, J.A.; de Jong, W.W. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: Hspb1-10. Cell Stress Chaperones 2003, 8, 53–61. [Google Scholar] [CrossRef]
- Vos, M.J.; Kanon, B.; Kampinga, H.H. Hspb7 is a sc35 speckle resident small heat shock protein. Biochim. Biophys. Acta 2009, 1793, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.P.; Benjamin, I.J. Small heat shock proteins: A new classification scheme in mammals. J. Mol. Cell. Cardiol. 2005, 38, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Bogomolovas, J.; Trexler, C.; Chen, J. The bag3-dependent and -independent roles of cardiac small heat shock proteins. JCI Insight 2019, 4, e126464. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, C.J.O.; Peters, C.; Schmid, P.W.N.; Stavropoulou, M.; Zou, J.; Dahiya, V.; Mymrikov, E.V.; Rockel, B.; Asami, S.; Haslbeck, M.; et al. The structure and oxidation of the eye lens chaperone alphaa-crystallin. Nat. Struct. Mol. Biol. 2019, 26, 1141–1150. [Google Scholar] [CrossRef]
- Braun, N.; Zacharias, M.; Peschek, J.; Kastenmuller, A.; Zou, J.; Hanzlik, M.; Haslbeck, M.; Rappsilber, J.; Buchner, J.; Weinkauf, S. Multiple molecular architectures of the eye lens chaperone alphab-crystallin elucidated by a triple hybrid approach. Proc. Natl. Acad. Sci. USA 2011, 108, 20491–20496. [Google Scholar] [CrossRef] [Green Version]
- Kriehuber, T.; Rattei, T.; Weinmaier, T.; Bepperling, A.; Haslbeck, M.; Buchner, J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010, 24, 3633–3642. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Sugiyama, Y.; Hayashi, Y.; Nyu-i, N.; Yoshida, M.; Nonaka, I.; Ishiura, S.; Arahata, K.; Ohno, S. Mkbp, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J. Cell Biol. 1998, 140, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, Y.; Suzuki, A.; Kishikawa, M.; Akutsu, R.; Hirose, T.; Waye, M.M.; Tsui, S.K.; Yoshida, S.; Ohno, S. Muscle develops a specific form of small heat shock protein complex composed of mkbp/hspb2 and hspb3 during myogenic differentiation. J. Biol. Chem. 2000, 275, 1095–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mymrikov, E.V.; Riedl, M.; Peters, C.; Weinkauf, S.; Haslbeck, M.; Buchner, J. Regulation of small heat-shock proteins by hetero-oligomer formation. J. Biol. Chem. 2020, 295, 158–169. [Google Scholar] [CrossRef]
- Zantema, A.; Verlaan-De Vries, M.; Maasdam, D.; Bol, S.; van der Eb, A. Heat shock protein 27 and alpha b-crystallin can form a complex, which dissociates by heat shock. J. Biol. Chem. 1992, 267, 12936–12941. [Google Scholar]
- Kato, K.; Shinohara, H.; Goto, S.; Inaguma, Y.; Morishita, R.; Asano, T. Copurification of small heat shock protein with alpha b crystallin from human skeletal muscle. J. Biol. Chem. 1992, 267, 7718–7725. [Google Scholar] [PubMed]
- Kato, K.; Goto, S.; Inaguma, Y.; Hasegawa, K.; Morishita, R.; Asano, T. Purification and characterization of a 20-kda protein that is highly homologous to alpha b crystallin. J. Biol. Chem. 1994, 269, 15302–15309. [Google Scholar]
- Delbecq, S.P.; Rosenbaum, J.C.; Klevit, R.E. A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry 2015, 54, 4276–4284. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, J.M.; Sun, X.; Benndorf, R.; Welsh, M.J. Interactions of hsp22 (hspb8) with hsp20, alphab-crystallin, and hspb3. Biochem. Biophys. Res. Commun. 2005, 337, 1006–1011. [Google Scholar] [CrossRef]
- Sun, X.; Fontaine, J.M.; Rest, J.S.; Shelden, E.A.; Welsh, M.J.; Benndorf, R. Interaction of human hsp22 (hspb8) with other small heat shock proteins. J. Biol. Chem. 2004, 279, 2394–2402. [Google Scholar] [CrossRef] [Green Version]
- Bukach, O.V.; Seit-Nebi, A.S.; Marston, S.B.; Gusev, N.B. Some properties of human small heat shock protein hsp20 (hspb6). Eur. J. Biochem. 2004, 271, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukach, O.V.; Glukhova, A.E.; Seit-Nebi, A.S.; Gusev, N.B. Heterooligomeric complexes formed by human small heat shock proteins hspb1 (hsp27) and hspb6 (hsp20). Biochim. Biophys. Acta 2009, 1794, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Heirbaut, M.; Lermyte, F.; Martin, E.M.; Beelen, S.; Verschueren, T.; Sobott, F.; Strelkov, S.V.; Weeks, S.D. The preferential heterodimerization of human small heat shock proteins hspb1 and hspb6 is dictated by the n-terminal domain. Arch. Biochem. Biophys. 2016, 610, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Heirbaut, M.; Lermyte, F.; Martin, E.M.; Beelen, S.; Sobott, F.; Strelkov, S.V.; Weeks, S.D. Specific sequences in the n-terminal domain of human small heat-shock protein hspb6 dictate preferential hetero-oligomerization with the orthologue hspb1. J. Biol. Chem. 2017, 292, 9944–9957. [Google Scholar] [CrossRef] [Green Version]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 2012, 17, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, A.P. Human small heat shock proteins: Protein interactomes of homo- and hetero-oligomeric complexes: An update. FEBS Lett. 2013, 587, 1959–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, G.K.; Benesch, J.L. Dynamical structure of alphab-crystallin. Prog. Biophys. Mol. Biol. 2014, 115, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hilton, G.R.; Lioe, H.; Stengel, F.; Baldwin, A.J.; Benesch, J.L. Small heat-shock proteins: Paramedics of the cell. Top. Curr. Chem. 2012, 328, 69–98. [Google Scholar]
- Jehle, S.; Vollmar, B.S.; Bardiaux, B.; Dove, K.K.; Rajagopal, P.; Gonen, T.; Oschkinat, H.; Klevit, R.E. N-terminal domain of alphab-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. USA 2011, 108, 6409–6414. [Google Scholar] [CrossRef] [Green Version]
- Muranova, L.K.; Weeks, S.D.; Strelkov, S.V.; Gusev, N.B. Characterization of mutants of human small heat shock protein hspb1 carrying replacements in the n-terminal domain and associated with hereditary motor neuron diseases. PLoS ONE 2015, 10, e0126248. [Google Scholar] [CrossRef] [Green Version]
- Clouser, A.F.; Baughman, H.E.; Basanta, B.; Guttman, M.; Nath, A.; Klevit, R.E. Interplay of disordered and ordered regions of a human small heat shock protein yields an ensemble of ‘quasi-ordered’ states. eLife 2019, 8, eLife50259. [Google Scholar] [CrossRef] [PubMed]
- Peschek, J.; Braun, N.; Rohrberg, J.; Back, K.C.; Kriehuber, T.; Kastenmuller, A.; Weinkauf, S.; Buchner, J. Regulated structural transitions unleash the chaperone activity of alphab-crystallin. Proc. Natl. Acad. Sci. USA 2013, 110, E3780–E3789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heirbaut, M.; Beelen, S.; Strelkov, S.V.; Weeks, S.D. Dissecting the functional role of the n-terminal domain of the human small heat shock protein hspb6. PLoS ONE 2014, 9, e105892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weeks, S.D.; Muranova, L.K.; Heirbaut, M.; Beelen, S.; Strelkov, S.V.; Gusev, N.B. Characterization of human small heat shock protein hspb1 alpha-crystallin domain localized mutants associated with hereditary motor neuron diseases. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatov, V.M.; Weeks, S.D.; Strelkov, S.V.; Gusev, N.B. The role of the arginine in the conserved n-terminal domain rlfdqxfg motif of human small heat shock proteins hspb1, hspb4, hspb5, hspb6, and hspb8. Int. J. Mol. Sci. 2018, 19, 2112. [Google Scholar] [CrossRef] [Green Version]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.R.; Vree Egberts, W.; Kondrat, F.D.L.; Hilton, G.R.; Ray, N.J.; Cole, A.R.; Carver, J.A.; Benesch, J.L.P.; Keep, N.H.; Boelens, W.C.; et al. Terminal regions confer plasticity to the tetrameric assembly of human hspb2 and hspb3. J. Mol. Biol. 2018, 430, 3297–3310. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J. Alpha-crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Clark, A.R.; Lubsen, N.H.; Slingsby, C. Shsp in the eye lens: Crystallin mutations, cataract and proteostasis. Int. J. Biochem. Cell Biol. 2012, 44, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, S.P.; Jehle, S.; Klevit, R. Binding determinants of the small heat shock protein, alphab-crystallin: Recognition of the ‘ixi’ motif. EMBO J. 2012, 31, 4587–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klevit, R.E. Peeking from behind the veil of enigma: Emerging insights on small heat shock protein structure and function. Cell Stress Chaperones 2020. [Google Scholar] [CrossRef]
- Nappi, L.; Aguda, A.H.; Nakouzi, N.A.; Lelj-Garolla, B.; Beraldi, E.; Lallous, N.; Thi, M.; Moore, S.; Fazli, L.; Battsogt, D.; et al. Ivermectin inhibits hsp27 and potentiates efficacy of oncogene targeting in tumor models. J. Clin. Investig. 2020, 130, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Datskevich, P.N.; Nefedova, V.V.; Sudnitsyna, M.V.; Gusev, N.B. Mutations of small heat shock proteins and human congenital diseases. Biochemistry 2012, 77, 1500–1514. [Google Scholar] [CrossRef] [PubMed]
- Muranova, L.K.; Ryzhavskaya, A.S.; Sudnitsyna, M.V.; Shatov, V.M.; Gusev, N.B. Small heat shock proteins and human neurodegenerative diseases. Biochemistry 2019, 84, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Bukach, O.V.; Seit-Nebi, A.S.; Gusev, N.B. The pivotal role of the beta 7 strand in the intersubunit contacts of different human small heat shock proteins. Cell Stress Chaperones 2010, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]









| WT HspB1 | R/A HspB1 | Δ HspB1 | WT HspB5 | R/A HspB5 | Δ HspB5 | ||
|---|---|---|---|---|---|---|---|
| WT HspB5 | Without heating | 0 | 0 | 0 | |||
| After heating | 1.00 (constant ~1.0) | 1.00 (constant ~1.0) | 1.00 (constant ~1.0) | ||||
| R/A HspB5 | Without heating | 0 | |||||
| After heating | 1.00 (constant ~1.0) | ||||||
| Δ HspB5 | Without heating | 0 | |||||
| After heating | 1.00 (constant ~1.0) | ||||||
| WT HspB6 | Without heating | 0.05 | 0.31 (variable 5–1) | 0.82 (variable 3–1) | 0.05 | 0.12 | 0 |
| After heating | 0.99 (constant ~1.1) | 0.99 (constant ~1.1) | 1.00 (constant ~1.2) | 0.99 (constant ~2.0) | 0.99 (variable 3–2) | 0.98 (variable 5–2) | |
| R/A HspB6 | Without heating | 0.04 | 0.12 | ||||
| After heating | 0.78 (variable 10–1) | 0.99 (variable 4–1) | |||||
| Δ HspB6 | Without heating | 0.05 | 0 | ||||
| After heating | 0.32 (variable 10–1) | 0.98 (variable 4–1) | |||||
| WT HspB8 | Without heating | 0 | 0 | 0 | 0 | 0 | 0 |
| After heating | 0 | 0 | 0 | 0 | 0 | 0 | |
| R/A HspB8 | Without heating | 0 | 0 | ||||
| After heating | 0 | 0 | |||||
| Δ HspB8 | Without heating | 0 | 0 | ||||
| After heating | 0 | 0 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatov, V.M.; Strelkov, S.V.; Gusev, N.B. The Heterooligomerization of Human Small Heat Shock Proteins Is Controlled by Conserved Motif Located in the N-Terminal Domain. Int. J. Mol. Sci. 2020, 21, 4248. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124248
Shatov VM, Strelkov SV, Gusev NB. The Heterooligomerization of Human Small Heat Shock Proteins Is Controlled by Conserved Motif Located in the N-Terminal Domain. International Journal of Molecular Sciences. 2020; 21(12):4248. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124248
Chicago/Turabian StyleShatov, Vladislav M., Sergei V. Strelkov, and Nikolai B. Gusev. 2020. "The Heterooligomerization of Human Small Heat Shock Proteins Is Controlled by Conserved Motif Located in the N-Terminal Domain" International Journal of Molecular Sciences 21, no. 12: 4248. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124248